
HL Paper 2
The periodic table shows the relationship between electron configuration and the properties of elements and is a valuable tool for making predictions in chemistry.
The ten elements in the first-row d-block have characteristic properties and many uses.
Define the term electronegativity.
(i) Outline two reasons why a sodium ion has a smaller radius than a sodium atom.
(ii) Explain why the ionic radius of \({{\text{P}}^{3 - }}\) is greater than the ionic radius of \({\text{S}}{{\text{i}}^{4 + }}\).
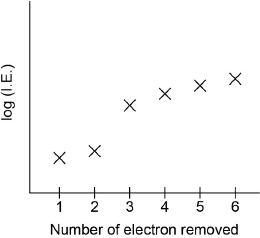
The graph below represents the successive ionization energies of sodium. The vertical axis plots log (ionization energy) instead of ionization energy to allow the data to be represented without using an unreasonably long vertical axis.
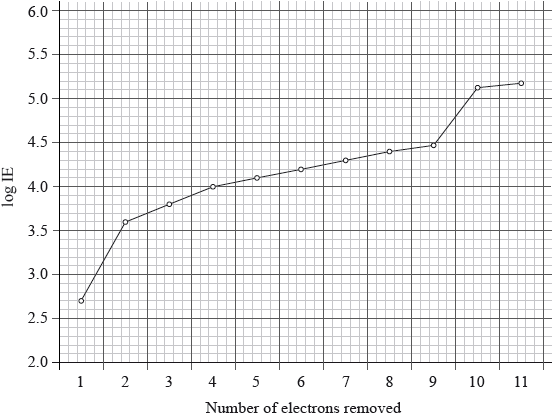
State the full electron configuration of sodium and explain how the successive ionization energy data for sodium are related to its electron configuration.
(i) Explain why the first ionization energy of aluminium is lower than the first ionization energy of magnesium.
(ii) Explain why the first ionization energy of sulfur is lower than the first ionization energy of phosphorus.
State and explain the type of reaction that takes place between \({\text{F}}{{\text{e}}^{3 + }}\) and \({{\text{H}}_{\text{2}}}{\text{O}}\) to form \({{\text{[Fe(}}{{\text{H}}_{\text{2}}}{\text{O}}{{\text{)}}_{\text{6}}}{\text{]}}^{3 + }}\) in terms of acid-base theories.
Explain why \({{\text{[Fe(}}{{\text{H}}_{\text{2}}}{\text{O}}{{\text{)}}_{\text{6}}}{\text{]}}^{3 + }}\) is coloured.
Outline the economic significance of the use of a catalyst in the Haber process which is an exothermic reaction.
Markscheme
power/strength/ability of an atom to attract electrons/shared electron pair / OWTTE;
in a (covalent) bond;
Accept the word “element” in place of “atom”.
Do not accept electron (singular).
(i) Na: 11 p, 11/ 2.8.1 \({{\text{e}}^ - }\) and \({\text{N}}{{\text{a}}^ + }\): 11 p, 10 / 2.8 \({{\text{e}}^ - }\) / same number of protons, less electrons / \({\text{N}}{{\text{a}}^ + }\) has 2 shells/energy levels, Na has 3 / OWTTE;
Na+: has greater net positive charge/same number of protons pulling smaller number of electrons;
(ii) Si4+: 10 \({{\text{e}}^ - }\) in 2 (filled) energy levels / electron arrangement 2.8 / OWTTE;
P3−: 18 \({{\text{e}}^ - }\) in 3 (filled) energy levels / electron arrangement 2.8.8, thus larger / OWTTE;
OR
\({\text{S}}{{\text{i}}^{4 + }}\): has 2 energy levels where as \({{\text{P}}^{3 - }}\) has 3/ \({{\text{P}}^{3 - }}\) has one more (filled) energy
level;
\({\text{S}}{{\text{i}}^{4 + }}\): 10 \({{\text{e}}^ - }\) in 2 energy levels where as \({{\text{P}}^{3 - }}\) has 18 \({{\text{e}}^ - }\), thus larger;
\({\text{1}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{s}}^{\text{1}}}\);
Do not accept [Ne] 3s1.
first electron easy/easiest to remove / 1 electron in outermost/\({\text{n}} = 3\) energy level;
large increase between 1st and 2nd IE as electron now removed from \({\text{n}} = 2\) / next 8 electrons more difficult to remove / show (relatively) small increase as these electrons are in the same energy level/second energy level/\({\text{n}} = 2\);
large increase between 9th and 10th IE as electron now removed from n = 1 / 2
electrons very hard/most difficult to remove / innermost/lowest/closest to the nucleus/energy level/\({\text{n}} = 1\) / OWTTE;
electron 11 also comes from 1s, so shows a small increase;
(i) outer electron in Al is in 3p/p orbital/sub-shell/sub-level;
higher orbital/sub-shell / e– further from nucleus / shielded by 3s electrons;
(ii) in S, electron paired in 3p/p orbital/sub-shell/sub-level;
Accept extra stability associated with half filled p sub-shell (in P).
repulsion between paired electrons (and therefore easier to remove);
Lewis acid-base (reaction);
\({{\text{H}}_{\text{2}}}{\text{O}}\): e-pair donor, \({\text{F}}{{\text{e}}^{3 + }}\): \({{\text{e}}^ - }\) pair acceptor / \({{\text{H}}_{\text{2}}}{\text{O}}\) donates an electron pair to \({\text{F}}{{\text{e}}^{3 + }}\);
d sub-levels are split into two sets of orbitals (of different energies);
electron transitions between (d) orbitals of different energies / d-d transition(s);
transmitted (visible) light is complementary colour;
(exothermic reactions) low temperature/less energy increases ammonia yield;
(iron) catalyst used to increase rate of reaction / equilibrium reached faster / same yield but produced faster/in shorter/less time;
Examiners report
Generally, the definition of electronegativity was good, but some made the error of saying that it was the attraction of one electron only; others did not specify that it is the ability of an atom to attract a shared electron pair in a covalent bond.
Reasons why a sodium ion has a smaller radius than a sodium atom solicited incomplete answers. The answer requires the number of shells, electrons and protons of both the ion and the atom. Many candidates correctly said that \({\text{N}}{{\text{a}}^ + }\) had the same number of protons but one electron less so the pulling effect on the electrons was greater. Not many candidates gave the electronic structure or number of shells of the two ions, \({{\text{P}}^{3 - }}\) and \({\text{S}}{{\text{i}}^{4 + }}\), to explain their difference in ionic radius.
The graphical question on successive ionization energies of sodium was well answered by many. Typically, they explained how the successive ionization energies of sodium are related to its electron configuration from the data given. Most candidates realized that aluminium’s outer electron is in the 3p orbital so further from the nucleus and thus easier to ionize than magnesium. Similarly, sulfur has a paired electron in the 3p sub-shell and the repulsion between paired electrons is greater than in phosphorus which has a half filled p sub-shell.
Many candidates did not give sufficient answers to the part on transition elements. Some realised that it was a Lewis acid-base reaction where the electrons are donated by the water molecule to \({\text{F}}{{\text{e}}^{3 + }}\). Explanations given for the colour of complex ions continue to be muddled and the language used imprecise. Many wrote of “a split d orbital” rather than the d sub-level being split into two sets of orbitals (of different energies). The colour seen was often attributed to electrons emitting those wavelengths in transitions from higher energy to lower energy d orbitals rather than the transmitted visible light being the complementary colour of the one absorbed.
Few complete answers were given about economic significance of the use of a catalyst in the Haber process. A point that was missing often was that because the reaction is exothermic the forward reaction would be favoured (and the yield) if the temperature is lowered, but this would bring about a slower reaction so a catalyst is necessary to reach the equilibrium faster. However, there were misconceptions both in as far as catalysts and energetic is concerned. It was surprising to see the number of candidates who referred to activation energy but used the concept incorrectly. Few candidates established a connection with equilibrium.
Few complete answers were given about economic significance of the use of a catalyst in the Haber process. A point that was missing often was that because the reaction is exothermic the forward reaction would be favoured (and the yield) if the temperature is lowered, but this would bring about a slower reaction so a catalyst is necessary to reach the equilibrium faster. However, there were misconceptions both in as far as catalysts and energetic is concerned. It was surprising to see the number of candidates who referred to activation energy but used the concept incorrectly. Few candidates established a connection with equilibrium.
Few complete answers were given about economic significance of the use of a catalyst in the Haber process. A point that was missing often was that because the reaction is exothermic the forward reaction would be favoured (and the yield) if the temperature is lowered, but this would bring about a slower reaction so a catalyst is necessary to reach the equilibrium faster. However, there were misconceptions both in as far as catalysts and energetic is concerned. It was surprising to see the number of candidates who referred to activation energy but used the concept incorrectly. Few candidates established a connection with equilibrium.
The emission spectrum of an element can be used to identify it.
Hydrogen spectral data give the frequency of 3.28 × 1015 s−1 for its convergence limit.
Calculate the ionization energy, in J, for a single atom of hydrogen using sections 1 and 2 of the data booklet.
Calculate the wavelength, in m, for the electron transition corresponding to the frequency in (a)(iii) using section 1 of the data booklet.
Deduce any change in the colour of the electrolyte during electrolysis.
Deduce the gas formed at the anode (positive electrode) when graphite is used in place of copper.
Explain why transition metals exhibit variable oxidation states in contrast to alkali metals.
Markscheme
IE «= ΔE = hν = 6.63 × 10–34 J s × 3.28 × 1015 s–1» = 2.17 × 10–18 «J»
[1 mark]
«\(\lambda = \frac{C}{{\text{v}}} = \frac{{3.00 \times {{10}^8}{\text{ m}}{{\text{s}}^{ - 1}}}}{{3.28 \times {{10}^{15}}{\text{ }}{{\text{s}}^{ - 1}}}} = \)» 9.15 × 10–8 «m»
[1 mark]
no change «in colour»
Do not accept “solution around cathode will become paler and solution around the anode will become darker”.
[1 mark]
oxygen/O2
Accept “carbon dioxide/CO2”.
[1 mark]
Transition metals:
«contain» d and s orbitals «which are close in energy»
OR
«successive» ionization energies increase gradually
Alkali metals:
second electron removed from «much» lower energy level
OR
removal of second electron requires large increase in ionization energy
[2 marks]
Examiners report
Copper is a metal that has been used by humans for thousands of years.
State the full electron configuration of \(^{{\text{65}}}{\text{Cu}}\).
State one difference in the physical properties of the isotopes \(^{{\text{63}}}{\text{Cu}}\) and \(^{{\text{65}}}{\text{Cu}}\) and explain why their chemical properties are the same.
Physical:
Chemical:
Describe the bonding in solid copper.
Markscheme
\({\text{1}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{s}}^{\text{2}}}{\text{3}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{d}}^{{\text{10}}}}{\text{4}}{{\text{s}}^{\text{1}}}{\text{/1}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{s}}^{\text{2}}}{\text{3}}{{\text{p}}^{\text{6}}}{\text{4}}{{\text{s}}^{{\text{13}}}}{{\text{d}}^{{\text{10}}}}\);
Physical:
\(^{{\text{63}}}{\text{Cu}}\) lower boiling point/melting point/density/greater rate of diffusion than \(^{{\text{65}}}{\text{Cu}}\);
Accept converse argument.
Do not accept “different mass”.
Chemical:
(properties identical because) same electron configuration/arrangement of electrons;
Accept “same number of protons and electrons”.
Do not accept “same number of electrons” OR “same valence (electrons)” OR “same atomic number” only.
electrostatic attraction;
between (a lattice of) cations/positive ions and delocalized/sea of electrons;
Do not award any mark for only stating “metallic bonding”.
Examiners report
Most were able to quote the electron configuration of copper correctly; but some gave [Ar] \({\text{4}}{{\text{s}}^{\text{1}}}\,{\text{3}}{{\text{d}}^{\text{9}}}\) when they were specifically asked for the full configuration. A few, inevitably gave \({\text{3}}{{\text{d}}^{\text{9}}}\,{\text{4}}{{\text{s}}^{\text{2}}}\). In (b), few related the difference in mass to a property and most did not give the comparison; “the same number of electrons and protons” was more popular than “the same electron configuration”. The descriptions of metallic bonding were disappointing; the mark for electrostatic attraction was rarely scored and many confused “nuclei” with “cations/positive ions”.
Most were able to quote the electron configuration of copper correctly; but some gave [Ar] \({\text{4}}{{\text{s}}^{\text{1}}}\,{\text{3}}{{\text{d}}^{\text{9}}}\) when they were specifically asked for the full configuration. A few, inevitably gave \({\text{3}}{{\text{d}}^{\text{9}}}\,{\text{4}}{{\text{s}}^{\text{2}}}\). In (b), few related the difference in mass to a property and most did not give the comparison; “the same number of electrons and protons” was more popular than “the same electron configuration”. The descriptions of metallic bonding were disappointing; the mark for electrostatic attraction was rarely scored and many confused “nuclei” with “cations/positive ions”.
Most were able to quote the electron configuration of copper correctly; but some gave [Ar] \({\text{4}}{{\text{s}}^{\text{1}}}\,{\text{3}}{{\text{d}}^{\text{9}}}\) when they were specifically asked for the full configuration. A few, inevitably gave \({\text{3}}{{\text{d}}^{\text{9}}}\,{\text{4}}{{\text{s}}^{\text{2}}}\). In (b), few related the difference in mass to a property and most did not give the comparison; “the same number of electrons and protons” was more popular than “the same electron configuration”. The descriptions of metallic bonding were disappointing; the mark for electrostatic attraction was rarely scored and many confused “nuclei” with “cations/positive ions”.
Bonds can be formed in many ways.
Bonds can be formed in many ways.
The equilibrium for a mixture of NO2 and N2O4 gases is represented as:
2NO2(g) \( \rightleftharpoons \) N2O4(g)
At 100°C, the equilibrium constant, Kc, is 0.21.
Discuss the bonding in the resonance structures of ozone.
Deduce one resonance structure of ozone and the corresponding formal charges on each oxygen atom.
The first six ionization energies, in kJ mol–1, of an element are given below.

Explain the large increase in ionization energy from IE3 to IE4.
At a given time, the concentration of NO2(g) and N2O4(g) were 0.52 and \(0.10{\text{ mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) respectively.
Deduce, showing your reasoning, if the forward or the reverse reaction is favoured at this time.
Comment on the value of ΔG when the reaction quotient equals the equilibrium constant, Q = K.
Markscheme
lone pair on p orbital «of O atom» overlaps/delocalizes with pi electrons «from double bond»
both O–O bonds have equal bond length
OR
both O–O bonds have same/1.5 bond order
OR
both O–O are intermediate between O–O AND O=O
both O–O bonds have equal bond energy
Accept “p/pi/\(\pi \) electrons are delocalized/not localized”.
[3 marks]
ALTERNATIVE 1:
FC: –1 AND +1 AND 0
ALTERNATIVE 2:
FC: 0 AND +1 AND –1
Accept any combination of lines, dots or crosses to represent electrons.
Do not accept structure that represents 1.5 bonds.
Do not penalize missing lone pairs if already penalized in 3(b).
If resonance structure is incorrect, no ECF.
Any one of the structures with correct formal charges for [2 max].
[2 marks]
Any two of:
IE4: electron in lower/inner shell/energy level
OR
IE4: more stable/full electron shell
IE4: electron closer to nucleus
OR
IE4: electron more tightly held by nucleus
IE4: less shielding by complete inner shells
Accept “increase in effective nuclear charge” for M2.
[2 marks]
«Qc = \(\frac{{0.10}}{{{{0.52}^2}}}\) =» 0.37
reaction proceeds to the left/NO2(g) «until Q = Kc»
OR
reverse reaction «favoured»
Do not award M2 without a calculation for M1 but remember to apply ECF.
[2 marks]
ΔG = 0
reaction at equilibrium
OR
rate of forward and reverse reaction is the same
OR
constant macroscopic properties
[2 marks]
Examiners report
Magnesium, a reactive metal found in many common minerals, is also an essential nutrient for both plants and animals.
Successive ionization energies of magnesium are given in the table below.

Magnesium metal is mainly used as a component in lightweight alloys, particularly in combination with aluminium and titanium.
Magnesium is usually produced by the electrolysis of molten magnesium chloride.
Define the term first ionization energy.
(i) Explain why the second ionization energy is greater than the first ionization energy.
(ii) Explain why the third ionization energy is much greater than the second ionization energy.
Although magnesium is usually found as \({\text{M}}{{\text{g}}^{2 + }}\) in its compounds, it is possible to use the Born-Haber cycle to investigate the possibility of \({\text{M}}{{\text{g}}^ + }\) being able to form stable compounds.
Use the ionization energy data from part (b), along with the other data provided below, to determine the enthalpy change of formation of MgCl(s). Assume that, because \({\text{M}}{{\text{g}}^ + }\) would be similar in size to \({\text{N}}{{\text{a}}^ + }\), MgCl would have a similar lattice enthalpy to NaCl.
Enthalpy of atomization of Mg \( + 146{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\)
Bond enthalpy in \({\text{C}}{{\text{l}}_{\text{2}}}\) \( + 243{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\)
Electron affinity of Cl \( + 349{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\)
Lattice enthalpy of NaCl \( + 790{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\)
Consider the lattice enthalpies of \({\text{Mg}}{{\text{F}}_{\text{2}}}\), \({\text{MgC}}{{\text{l}}_2}\) and \({\text{CaC}}{{\text{l}}_{\text{2}}}\). List these from the most endothermic to the least endothermic and explain your order.
\({\text{Most endothermic}} \to {\text{Least endothermic}}\)
Magnesium hydroxide, \({\text{Mg(OH}}{{\text{)}}_{\text{2}}}\), is only sparingly soluble in water and the equilibrium below exists when excess solid is in contact with a saturated solution.
\[{\text{Mg(OH}}{{\text{)}}_2}{\text{(s)}} \rightleftharpoons {\text{M}}{{\text{g}}^{2 + }}{\text{(aq)}} + {\text{2O}}{{\text{H}}^ - }{\text{(aq)}}\]
Outline how the solubility of magnesium hydroxide will vary with pH.
(i) Describe the bonding present in magnesium metal.
(ii) Suggest why magnesium is harder than sodium.
(iii) Outline why alloys are generally less malleable than their component metals.
(i) Draw a labelled diagram of a suitable apparatus for the electrolysis.
(ii) State equations for the reactions that take place at the electrodes.
Negative electrode (cathode) reaction:
Positive electrode (anode) reaction:
(iii) When dilute aqueous magnesium chloride is used as the electrolyte, the reactions at both electrodes are different. State equations for the reactions that occur in aqueous solution.
Negative electrode (cathode) reaction:
Positive electrode (anode) reaction:
(iv) Outline why magnesium metal is not produced in the electrolysis of aqueous magnesium chloride.
Markscheme
minimum energy required to remove one electron / energy required to remove most loosely bound/outermost electron;
from gaseous/isolated atom;
Accept “gaseous state”.
More extensive definitions involving one mole may be given.
(i) electrons lost in same orbital/valence shell;
(second) electron/electron (being lost from \({\text{M}}{{\text{g}}^ + }\) is) closer to the nucleus;
(second) electron/electron (being lost from \({\text{M}}{{\text{g}}^ + }\)) not subject to e-e repulsion from others in same level;
Apply OWTTE for all marking points.
Do not accept “less electrons to share the charge” or answers employing this concept.
(ii) electron in lower energy level / more stable electron shell;
electron closer to nucleus;
less shielding by complete inner shells / increase in effective nuclear charge;
Apply OWTTE for all marking points.
\(\Delta {H_{{\text{at}}}}{\text{(Cl)}} = \frac{1}{2} \times 243{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Correct calculation of atomization enthalpy of Cl.
\(\Delta {H_{\text{f}}} = + 146 + \frac{1}{2}243 + 738 + ( - 349) + ( - 790)\);
Correct sign and magnitude of all terms.
\( = - {\text{134 (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Award [3] for correct final answer.
Final mark involves correct computation of equation the student has produced.
Award [2] for –12 (bond enthalpy of Cl not halved) or +134 (signs wrong).
Award [1] for +12 (bond enthalpy of Cl not halved and signs wrong).
\({\text{Mg}}{{\text{F}}_2}\) –\({\text{MgC}}{{\text{l}}_2}\) –\({\text{CaC}}{{\text{l}}_2}\);
\({{\text{F}}^ - }\) smaller (ionic radius) than \({\text{C}}{{\text{l}}^ - }\) / \({\text{C}}{{\text{l}}^ - }\) larger (ionic radius) than \({{\text{F}}^ - }\);
\({\text{M}}{{\text{g}}^{2 + }}\) smaller (ionic radius) than \({\text{C}}{{\text{a}}^{2 + }}\) / \({\text{C}}{{\text{a}}^{2 + }}\) larger (ionic radius) than \({\text{M}}{{\text{g}}^{2 + }}\);
Accept use of atomic radius rather than ionic radius.
more soluble at low pH / less soluble at high pH;
higher pH / \({\text{O}}{{\text{H}}^ - }\) will shift the equilibrium to the left / lower pH / \({{\text{H}}^ + }\) will (react with \({\text{O}}{{\text{H}}^ - }\) and) shift the equilibrium to the right;
(i) lattice/layers/framework of cations/magnesium ions/\({\text{M}}{{\text{g}}^{2 + }}\);
surrounded by delocalized electrons / in a sea/flux of delocalized electrons;
Accept “mobile” instead of “delocalized”.
(ii) Mg has more delocalized electrons (than Na);
Accept “Mg has more valence electrons than Na” / “Mg is Mg2+ but Na is only Na+”.
(iii) layers of ions/atoms/particles cannot slide over each other so easily (as different sized ions/atoms/particles) / OWTTE;
(i) 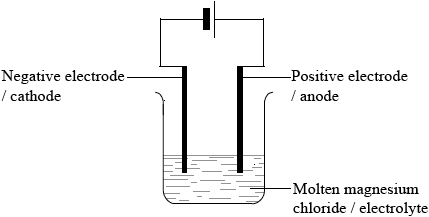
Diagram:
two electrodes connected to a power pack/battery and immersed in an electrolyte;
Do not award mark if salt bridge included in diagram.
Labelling:
anode/positive electrode, cathode/negative electrode, molten magnesium chloride/MgCl2 (l)/electrolyte correctly labelled;
Check candidates know which end of a battery symbol is which charge.
(ii) Negative electrode (cathode): \({\text{M}}{{\text{g}}^{2 + }}{\text{(l)}} + {\text{2}}{{\text{e}}^ - } \to {\text{Mg (s)}}\);
Positive electrode (anode): \[{\text{2C}}{{\text{l}}^ - }{\text{(l)}} \to {\text{C}}{{\text{l}}_2}{\text{(g)}} + {\text{2}}{{\text{e}}^ - }\];
Accept \(C{l^ - }(l) \to \frac{1}{2}C{l_2}(g) + {e^ - }\).
Ignore state symbols.
Allow e instead of e–.
If both correct equations are given for the wrong electrodes award [1 max].
(iii) Negative electrode (cathode):
\({\text{2}}{{\text{H}}_2}{\text{O(l)}} + {\text{2}}{{\text{e}}^ - } \to {{\text{H}}_2}{\text{(g)}} + {\text{2O}}{{\text{H}}^ - }{\text{(aq)}}/{\text{2}}{{\text{H}}^ + }{\text{(aq)}} + {\text{2}}{{\text{e}}^ - } \to {{\text{H}}_2}{\text{(g)}}\);
Accept \(4{H_2}O(l) + 4{e^ - } \to 2{H_2}(g) + 4O{H^ - }(aq) / 4{H^ + }(aq) + 4{e^ - } \to 2{H_2}(g)\) / \({H_2}O(l) + {e^ - } \to \frac{1}{2}{H_2}(g) + O{H^ - }(aq)/{H^ + }(aq) + {e^ - } \to \frac{1}{2}{H_2}(g)\).
Positive electrode (anode):
\({\text{2}}{{\text{H}}_2}{\text{O(l)}} \to {{\text{O}}_2}{\text{(g)}} + {\text{4}}{{\text{H}}^ + }{\text{(aq)}} + {\text{4}}{{\text{e}}^ - }/{\text{4O}}{{\text{H}}^ - }{\text{(aq)}} \to {{\text{O}}_2}{\text{(g)}} + {\text{2}}{{\text{H}}_2}{\text{O(l)}} + {\text{4}}{{\text{e}}^ - }\);
Accept \({H_2}O(l) \to \frac{1}{2}{O_2}(g) + 2{H^ + }(aq) + 2{e^ - } / 2O{H^ - }(aq) \to \frac{1}{2}{O_2}(g) + {H_2}O(l) + 2{e^ - }\).
State symbols not required.
Allow e instead of e–.
If both correct equations are given for the wrong electrodes award [1 max].
(iv) water/hydrogen ions more easily reduced/better oxidizing agents/have a more positive \({E^\Theta }\) (than magnesium ions);
Accept converse statements for magnesium ions.
Accept “magnesium is very reactive/high in reactivity series” / OWTTE.
Examiners report
This was the most popular of the Section B questions, which was surprising because it was often not well answered. Most students were aware of the change involved in ionization, some realised this relates to the most easily lost electron, some that it refers to gas phase changes and a few both. Explanations of the changes in the values of successive ionization energies in terms of the attraction of the nucleus and the repulsion from other electrons were generally weak, however candidates quite often recognised that the third electron lost had to come from a more stable electron energy level. Very few were able to correctly sum the enthalpy terms involved in the Born-Haber cycle, in addition candidates rarely halved the bond enthalpy of chlorine and a significant number appeared not to realise the question referred to MgCl rather than \({\text{MgC}}{{\text{l}}_2}\). There were some G2 comments that space should have been left for students to draw a cycle, but this would have implied that such a drawing was required, hence it is better to train students to use additional paper for any background support they may require. Quite a lot of students correctly deduced the order of lattice enthalpy of the group (II) halides, and a number correctly explained this in terms of ionic radii, though many incorrectly invoked electronegativities. Many students could correctly explain the effect of pH on the solubility of \({\text{Mg(OH}}{{\text{)}}_{\text{2}}}\), though a significant number attempted to answer the opposite question; how pH would depend on the concentration of dissolved \({\text{Mg(OH}}{{\text{)}}_{\text{2}}}\)! It was surprising how few students could correctly describe metallic bonding, how it is affected by the number of delocalized electrons per cation and the way it leads to malleability. Alloys are not specifically mentioned in the syllabus but the mark scheme gave credit for answers that indicated the student was aware that malleability is associated with layers of metal atoms/cations sliding over each other. Most students were able to draw appropriately labelled diagrams of electrolysis apparatus, though sometimes the labelling of the polarity of the electrodes did not correspond to the polarity of the battery symbol drawn. Quite a few candidates could quote equations for the reactions occurring the electrodes in both the liquid and aqueous state; both seemed to be equally well answered which was perhaps a little surprisingly. Many could also give good explanations as to why electrolysis of the aqueous solution did not produce magnesium metal, though confusions in terminology (such as hydrogen rather than hydrogen ions being reduced) were not uncommon.
This was the most popular of the Section B questions, which was surprising because it was often not well answered. Most students were aware of the change involved in ionization, some realised this relates to the most easily lost electron, some that it refers to gas phase changes and a few both. Explanations of the changes in the values of successive ionization energies in terms of the attraction of the nucleus and the repulsion from other electrons were generally weak, however candidates quite often recognised that the third electron lost had to come from a more stable electron energy level. Very few were able to correctly sum the enthalpy terms involved in the Born-Haber cycle, in addition candidates rarely halved the bond enthalpy of chlorine and a significant number appeared not to realise the question referred to MgCl rather than \({\text{MgC}}{{\text{l}}_2}\). There were some G2 comments that space should have been left for students to draw a cycle, but this would have implied that such a drawing was required, hence it is better to train students to use additional paper for any background support they may require. Quite a lot of students correctly deduced the order of lattice enthalpy of the group (II) halides, and a number correctly explained this in terms of ionic radii, though many incorrectly invoked electronegativities. Many students could correctly explain the effect of pH on the solubility of \({\text{Mg(OH}}{{\text{)}}_{\text{2}}}\), though a significant number attempted to answer the opposite question; how pH would depend on the concentration of dissolved \({\text{Mg(OH}}{{\text{)}}_{\text{2}}}\)! It was surprising how few students could correctly describe metallic bonding, how it is affected by the number of delocalized electrons per cation and the way it leads to malleability. Alloys are not specifically mentioned in the syllabus but the mark scheme gave credit for answers that indicated the student was aware that malleability is associated with layers of metal atoms/cations sliding over each other. Most students were able to draw appropriately labelled diagrams of electrolysis apparatus, though sometimes the labelling of the polarity of the electrodes did not correspond to the polarity of the battery symbol drawn. Quite a few candidates could quote equations for the reactions occurring the electrodes in both the liquid and aqueous state; both seemed to be equally well answered which was perhaps a little surprisingly. Many could also give good explanations as to why electrolysis of the aqueous solution did not produce magnesium metal, though confusions in terminology (such as hydrogen rather than hydrogen ions being reduced) were not uncommon.
This was the most popular of the Section B questions, which was surprising because it was often not well answered. Most students were aware of the change involved in ionization, some realised this relates to the most easily lost electron, some that it refers to gas phase changes and a few both. Explanations of the changes in the values of successive ionization energies in terms of the attraction of the nucleus and the repulsion from other electrons were generally weak, however candidates quite often recognised that the third electron lost had to come from a more stable electron energy level. Very few were able to correctly sum the enthalpy terms involved in the Born-Haber cycle, in addition candidates rarely halved the bond enthalpy of chlorine and a significant number appeared not to realise the question referred to MgCl rather than \({\text{MgC}}{{\text{l}}_2}\). There were some G2 comments that space should have been left for students to draw a cycle, but this would have implied that such a drawing was required, hence it is better to train students to use additional paper for any background support they may require. Quite a lot of students correctly deduced the order of lattice enthalpy of the group (II) halides, and a number correctly explained this in terms of ionic radii, though many incorrectly invoked electronegativities. Many students could correctly explain the effect of pH on the solubility of \({\text{Mg(OH}}{{\text{)}}_{\text{2}}}\), though a significant number attempted to answer the opposite question; how pH would depend on the concentration of dissolved \({\text{Mg(OH}}{{\text{)}}_{\text{2}}}\)! It was surprising how few students could correctly describe metallic bonding, how it is affected by the number of delocalized electrons per cation and the way it leads to malleability. Alloys are not specifically mentioned in the syllabus but the mark scheme gave credit for answers that indicated the student was aware that malleability is associated with layers of metal atoms/cations sliding over each other. Most students were able to draw appropriately labelled diagrams of electrolysis apparatus, though sometimes the labelling of the polarity of the electrodes did not correspond to the polarity of the battery symbol drawn. Quite a few candidates could quote equations for the reactions occurring the electrodes in both the liquid and aqueous state; both seemed to be equally well answered which was perhaps a little surprisingly. Many could also give good explanations as to why electrolysis of the aqueous solution did not produce magnesium metal, though confusions in terminology (such as hydrogen rather than hydrogen ions being reduced) were not uncommon.
This was the most popular of the Section B questions, which was surprising because it was often not well answered. Most students were aware of the change involved in ionization, some realised this relates to the most easily lost electron, some that it refers to gas phase changes and a few both. Explanations of the changes in the values of successive ionization energies in terms of the attraction of the nucleus and the repulsion from other electrons were generally weak, however candidates quite often recognised that the third electron lost had to come from a more stable electron energy level. Very few were able to correctly sum the enthalpy terms involved in the Born-Haber cycle, in addition candidates rarely halved the bond enthalpy of chlorine and a significant number appeared not to realise the question referred to MgCl rather than \({\text{MgC}}{{\text{l}}_2}\). There were some G2 comments that space should have been left for students to draw a cycle, but this would have implied that such a drawing was required, hence it is better to train students to use additional paper for any background support they may require. Quite a lot of students correctly deduced the order of lattice enthalpy of the group (II) halides, and a number correctly explained this in terms of ionic radii, though many incorrectly invoked electronegativities. Many students could correctly explain the effect of pH on the solubility of \({\text{Mg(OH}}{{\text{)}}_{\text{2}}}\), though a significant number attempted to answer the opposite question; how pH would depend on the concentration of dissolved \({\text{Mg(OH}}{{\text{)}}_{\text{2}}}\)! It was surprising how few students could correctly describe metallic bonding, how it is affected by the number of delocalized electrons per cation and the way it leads to malleability. Alloys are not specifically mentioned in the syllabus but the mark scheme gave credit for answers that indicated the student was aware that malleability is associated with layers of metal atoms/cations sliding over each other. Most students were able to draw appropriately labelled diagrams of electrolysis apparatus, though sometimes the labelling of the polarity of the electrodes did not correspond to the polarity of the battery symbol drawn. Quite a few candidates could quote equations for the reactions occurring the electrodes in both the liquid and aqueous state; both seemed to be equally well answered which was perhaps a little surprisingly. Many could also give good explanations as to why electrolysis of the aqueous solution did not produce magnesium metal, though confusions in terminology (such as hydrogen rather than hydrogen ions being reduced) were not uncommon.
This was the most popular of the Section B questions, which was surprising because it was often not well answered. Most students were aware of the change involved in ionization, some realised this relates to the most easily lost electron, some that it refers to gas phase changes and a few both. Explanations of the changes in the values of successive ionization energies in terms of the attraction of the nucleus and the repulsion from other electrons were generally weak, however candidates quite often recognised that the third electron lost had to come from a more stable electron energy level. Very few were able to correctly sum the enthalpy terms involved in the Born-Haber cycle, in addition candidates rarely halved the bond enthalpy of chlorine and a significant number appeared not to realise the question referred to MgCl rather than \({\text{MgC}}{{\text{l}}_2}\). There were some G2 comments that space should have been left for students to draw a cycle, but this would have implied that such a drawing was required, hence it is better to train students to use additional paper for any background support they may require. Quite a lot of students correctly deduced the order of lattice enthalpy of the group (II) halides, and a number correctly explained this in terms of ionic radii, though many incorrectly invoked electronegativities. Many students could correctly explain the effect of pH on the solubility of \({\text{Mg(OH}}{{\text{)}}_{\text{2}}}\), though a significant number attempted to answer the opposite question; how pH would depend on the concentration of dissolved \({\text{Mg(OH}}{{\text{)}}_{\text{2}}}\)! It was surprising how few students could correctly describe metallic bonding, how it is affected by the number of delocalized electrons per cation and the way it leads to malleability. Alloys are not specifically mentioned in the syllabus but the mark scheme gave credit for answers that indicated the student was aware that malleability is associated with layers of metal atoms/cations sliding over each other. Most students were able to draw appropriately labelled diagrams of electrolysis apparatus, though sometimes the labelling of the polarity of the electrodes did not correspond to the polarity of the battery symbol drawn. Quite a few candidates could quote equations for the reactions occurring the electrodes in both the liquid and aqueous state; both seemed to be equally well answered which was perhaps a little surprisingly. Many could also give good explanations as to why electrolysis of the aqueous solution did not produce magnesium metal, though confusions in terminology (such as hydrogen rather than hydrogen ions being reduced) were not uncommon.
This was the most popular of the Section B questions, which was surprising because it was often not well answered. Most students were aware of the change involved in ionization, some realised this relates to the most easily lost electron, some that it refers to gas phase changes and a few both. Explanations of the changes in the values of successive ionization energies in terms of the attraction of the nucleus and the repulsion from other electrons were generally weak, however candidates quite often recognised that the third electron lost had to come from a more stable electron energy level. Very few were able to correctly sum the enthalpy terms involved in the Born-Haber cycle, in addition candidates rarely halved the bond enthalpy of chlorine and a significant number appeared not to realise the question referred to MgCl rather than \({\text{MgC}}{{\text{l}}_2}\). There were some G2 comments that space should have been left for students to draw a cycle, but this would have implied that such a drawing was required, hence it is better to train students to use additional paper for any background support they may require. Quite a lot of students correctly deduced the order of lattice enthalpy of the group (II) halides, and a number correctly explained this in terms of ionic radii, though many incorrectly invoked electronegativities. Many students could correctly explain the effect of pH on the solubility of \({\text{Mg(OH}}{{\text{)}}_{\text{2}}}\), though a significant number attempted to answer the opposite question; how pH would depend on the concentration of dissolved \({\text{Mg(OH}}{{\text{)}}_{\text{2}}}\)! It was surprising how few students could correctly describe metallic bonding, how it is affected by the number of delocalized electrons per cation and the way it leads to malleability. Alloys are not specifically mentioned in the syllabus but the mark scheme gave credit for answers that indicated the student was aware that malleability is associated with layers of metal atoms/cations sliding over each other. Most students were able to draw appropriately labelled diagrams of electrolysis apparatus, though sometimes the labelling of the polarity of the electrodes did not correspond to the polarity of the battery symbol drawn. Quite a few candidates could quote equations for the reactions occurring the electrodes in both the liquid and aqueous state; both seemed to be equally well answered which was perhaps a little surprisingly. Many could also give good explanations as to why electrolysis of the aqueous solution did not produce magnesium metal, though confusions in terminology (such as hydrogen rather than hydrogen ions being reduced) were not uncommon.
This was the most popular of the Section B questions, which was surprising because it was often not well answered. Most students were aware of the change involved in ionization, some realised this relates to the most easily lost electron, some that it refers to gas phase changes and a few both. Explanations of the changes in the values of successive ionization energies in terms of the attraction of the nucleus and the repulsion from other electrons were generally weak, however candidates quite often recognised that the third electron lost had to come from a more stable electron energy level. Very few were able to correctly sum the enthalpy terms involved in the Born-Haber cycle, in addition candidates rarely halved the bond enthalpy of chlorine and a significant number appeared not to realise the question referred to MgCl rather than \({\text{MgC}}{{\text{l}}_2}\). There were some G2 comments that space should have been left for students to draw a cycle, but this would have implied that such a drawing was required, hence it is better to train students to use additional paper for any background support they may require. Quite a lot of students correctly deduced the order of lattice enthalpy of the group (II) halides, and a number correctly explained this in terms of ionic radii, though many incorrectly invoked electronegativities. Many students could correctly explain the effect of pH on the solubility of \({\text{Mg(OH}}{{\text{)}}_{\text{2}}}\), though a significant number attempted to answer the opposite question; how pH would depend on the concentration of dissolved \({\text{Mg(OH}}{{\text{)}}_{\text{2}}}\)! It was surprising how few students could correctly describe metallic bonding, how it is affected by the number of delocalized electrons per cation and the way it leads to malleability. Alloys are not specifically mentioned in the syllabus but the mark scheme gave credit for answers that indicated the student was aware that malleability is associated with layers of metal atoms/cations sliding over each other. Most students were able to draw appropriately labelled diagrams of electrolysis apparatus, though sometimes the labelling of the polarity of the electrodes did not correspond to the polarity of the battery symbol drawn. Quite a few candidates could quote equations for the reactions occurring the electrodes in both the liquid and aqueous state; both seemed to be equally well answered which was perhaps a little surprisingly. Many could also give good explanations as to why electrolysis of the aqueous solution did not produce magnesium metal, though confusions in terminology (such as hydrogen rather than hydrogen ions being reduced) were not uncommon.
A sample of magnesium contains three isotopes: magnesium-24, magnesium-25 and magnesium-26, with abundances of 77.44%, 10.00% and 12.56% respectively.
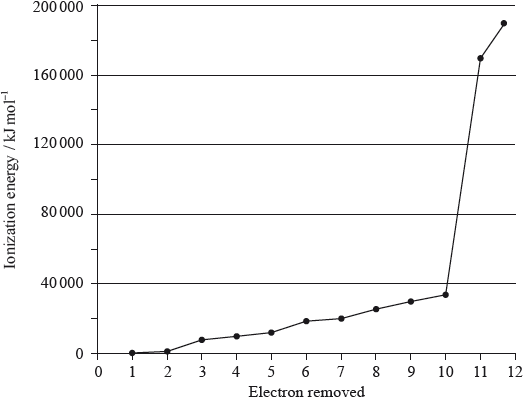
A graph of the successive ionization energies of magnesium is shown below.
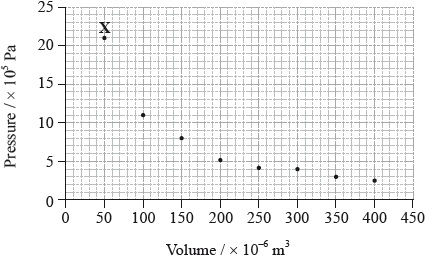
The graph below shows pressure and volume data collected for a sample of carbon dioxide gas at 330 K.
(i) Calculate the relative atomic mass of this sample of magnesium correct to two decimal places.
(ii) Predict the relative atomic radii of the three magnesium isotopes, giving your reasons.
(i) Explain the increase in ionization energy values from the 3rd to the 8th electrons.
(ii) Explain the sharp increase in ionization energy values between the 10th and 11th electrons.
(i) Magnesium reacts with oxygen to form an ionic compound, magnesium oxide. Describe how the ions are formed, and the structure and bonding in magnesium oxide.
(ii) Carbon reacts with oxygen to form a covalent compound, carbon dioxide. Describe what is meant by a covalent bond.
(iii) State why magnesium and oxygen form an ionic compound while carbon and oxygen form a covalent compound.
(i) Predict the type of hybridization of the carbon and oxygen atoms in \({\text{C}}{{\text{O}}_{\text{2}}}\).
(ii) Sketch the orbitals of an oxygen atom in \({\text{C}}{{\text{O}}_{\text{2}}}\) on the energy level diagram provided, including the electrons that occupy each orbital.

(iii) Define the term electronegativity.
(iv) Explain why oxygen has a larger electronegativity than carbon.
(i) Draw a best-fit curve for the data on the graph.
(ii) Use the data point labelled X to determine the amount, in mol, of carbon dioxide gas in the sample.
(i) Most indicators are weak acids. Describe qualitatively how indicators work.
(ii) Identify a suitable indicator for a titration between a weak acid and a strong base, using Table 16 of the Data Booklet.
Markscheme
(i) \(\left( {\frac{{(77.44 \times 24) + (10.00 \times 25) + (12.56 \times 26)}}{{100}}} \right)\);
24.35;
Award [2] for correct final answer.
Two decimal places are required for M2.
Do not award any marks for 24.31 without showing method (as the value can be copied from the Data Booklet).
(ii) same atomic radii / 160 pm;
isotopes only differ by number of neutrons/size of nucleus / radius determined by electron shells and number of protons / OWTTE;
Accept neutrons do not affect distance of electrons / OWTTE.
(i) decreasing repulsion between electrons / radius decreases as electrons are removed;
Accept increasing positive charge on ion attracts electrons more strongly.
(ii) 10th electron is in second energy level/shell while 11th electron is in first energy level/shell / 10th is removing electron from electronic arrangement 2,1 while 11th ionization energy is removing electron from electronic arrangement 2;
11th electron removed is much closer to the nucleus / 11th electron removed from a (much) lower energy level/shell;
Accept opposite statement for 10th electron.
(i) magnesium (atom) gives two electrons to oxygen (atom) / oxygen (atom) takes two electrons from magnesium (atom) / magnesium (atom) loses two electrons and oxygen (atom) gains two electrons;
3-dimensional/3-D arrangement of ions / lattice of ions;
(electrostatic) attraction between oppositely charged ions/\({\text{M}}{{\text{g}}^{2 + }}\) and \({{\text{O}}^{2 - }}\);
(ii) electrostatic attraction between a pair of electrons and (positively charged) nuclei;
Accept a/two pairs of shared electrons.
(iii) difference in electronegativity is larger between Mg and O/smaller between C and O;
Accept reference to a numerical value of difference in electronegativity such as above and below 1.80.
(i) C: sp hybridization;
O: \({\text{s}}{{\text{p}}^{\text{2}}}\) hybridization;
Award [1] if the answer is sp without specifying C or O atoms.
(ii) 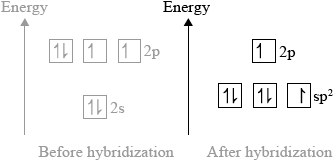
three \({\text{s}}{{\text{p}}^{\text{2}}}\) orbitals and one p-orbital at higher energy;
\({\text{s}}{{\text{p}}^{\text{2}}}\) orbitals contain: two, two and one electron and p-orbital contains one electron;
Do not allow ECF from (d)(i).
(iii) ability of atom/nucleus to attract bonding/shared pair of electrons / attraction of nucleus for bonding/shared pair of electrons / OWTTE;
(iv) (same number of shells but) increase in nuclear charge/atomic number/number of protons increases electronegativity / O has more protons than C;
Accept oxygen has a higher effective nuclear charge.
decrease in radius along the period increases electronegativity / O has smaller radius than C;
(i) smooth curve through the data;
Do not accept a curve that passes through all of the points or an answer that joins the points using lines.
(ii) \(p = 21 \times {10^5}/2.1 \times {10^6}{\text{ (Pa)}}/2.1 \times {10^3}{\text{ (kPa)}}\) and
\(V = 50 \times {10^{ - 6}}/5.0 \times {10^{ - 5}}{\text{ }}({{\text{m}}^3})/5.0 \times {10^{ - 2}}{\text{ }}({\text{d}}{{\text{m}}^3})\);
\(\left( {n = \frac{{pV}}{{RT}}} \right)\frac{{2.1 \times {{10}^6} \times 5.0 \times {{10}^{ - 5}}}}{{8.31 \times 330}}\);
\(n = 0.038{\text{ (mol)}}\);
Award [3] for correct final answer.
For M3 apply ECF for correct computation of the equation the student has written, unless more than one mistake is made prior this point.
(i) equilibrium between HIn and \({\text{I}}{{\text{n}}^ - }/{\text{HIn}} \rightleftharpoons {\text{I}}{{\text{n}}^ - } + {{\text{H}}^ + }\);
the colours of HIn and \({\text{I}}{{\text{n}}^ - }\) are different;
if added to acid, the equilibrium shifts to the left and the colour of HIn is seen / OWTTE;
if added to base/alkali, the equilibrium shifts to the right and the colour of \({\text{I}}{{\text{n}}^ - }\) is seen / OWTTE;
(ii) phenolphthalein;
Accept phenol red.
Examiners report
(i) Most candidates were able to calculate the relative atomic mass to the correct number of decimal places.
(ii) Only strong candidates were able to predict the same radius for the isotopes and gave correct reasoning. However, the majority of candidates predicted that a larger number of neutrons resulted is a smaller radius, reflecting a poor understanding of atomic structure.
(i) Very few candidates were able to explain the increase in successive ionization energies for electrons removed from the same sub-shell. Many candidates gave incorrect reasoning.
(ii) The increase between the 10th and 11th ionization energies of magnesium was explained correctly by about half of the candidates. Few candidates scored the first mark by identifying the correct shells or sub-shells the electrons are removed from.
(i) Well answered by many candidates. A few candidates were confusing ionic with covalent bonding, and some referred to a linear MgO molecule in an ionic lattice.
(ii) Few candidates were able to describe the covalent bond precisely. Those who didn’t score usually didn’t make any reference to pairs of electrons.
(iii) Many candidates obtained this mark with satisfactory arguments. It was disappointing to see the abundance of answers based on “is a metal with a non-metal” or “both are non-metals”.
(i) A few candidates identified sp hybridization based on a linear structure. Only the strongest candidates were able to give the correct hybridization for oxygen as well.
(ii) This was the most challenging question on the paper. It was rare to see a correct answer. It seems candidates did not have a good understanding of hybridization.
(iii) Less than half the candidates were able to define electronegativity precisely. Many candidates did not relate it to the pair of electrons in a covalent bond, and simply talked about attracting electrons, which was not sufficient for the mark.
(iv) Many candidates gained the first mark by stating that oxygen has more protons than carbon. But very few candidates identified the second factor, which is the smaller radius of oxygen.
(i) More than half of the candidates drew a smooth curve that was central to the data points. Errors included straight lines, curves joining all data points, or a curve that was not central to the points.
(ii) A very well answered question. Some candidates converted the units of p and V incorrectly and others did not read the scales of the graph correctly.
(i) Many candidates could explain the behaviour of indicators, but there were also some poor answers that did not acknowledge the importance of equilibrium in the action of an indicator.
(ii) Most candidates suggested a suitable indicator.
Lithium and boron are elements in period 2 of the periodic table. Lithium occurs in group 1 (the alkali metals) and boron occurs in group 3. Isotopes exist for both elements.
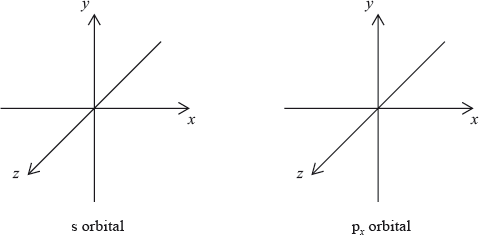
The electron configuration of boron is \({\text{1}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^{\text{1}}}\). Draw the shape of an s orbital and a \({{\text{p}}_x}\) orbital on the axes below.
(ii) Cobalt is a transition metal. One common ion of cobalt is \({\text{C}}{{\text{o}}^{3 + }}\). Draw the orbital diagram (using the arrow-in-box notation) for the \({\text{C}}{{\text{o}}^{3 + }}\) ion.

(iii) State the other most common ion of cobalt.
(iv) Explain why the complex \({\text{[Co(N}}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{6}}}{\text{]C}}{{\text{l}}_{\text{3}}}\) is coloured.
Markscheme
symmetrical shape of s orbital and dumbbell-shaped p orbital with electron density along x-axis;
(ii) 
Allow full arrows instead of half-arrows for example \( \uparrow \downarrow \).
Do not allow arrows with the same spin for example \( \uparrow \uparrow \) or \( \downarrow \downarrow \) in the same orbital.
Do not allow an orbital diagram with a \(4{s^1}3{d^5}\) configuration.
(iii) \({\text{C}}{{\text{o}}^{2 + }}\);
Accept +2, 2+, cobalt(II), II.
(iv) partially filled/incomplete d subshell/sub-level/orbitals;
d orbitals split (into two sets of different energies);
(colour due to) electron transition between (split) d orbitals / d to d transitions / frequencies of visible light absorbed by electrons moving from lower to higher d levels ;
colour due to remaining frequencies / complementary colour seen;
Allow wavelength as well as frequency.
Examiners report
In part (iii), a common mistake involved candidates drawing the lobe of electron density around the y or z axes for the \({{\text{p}}_x}\) orbital. Some candidates drew three dumbells for the s-orbital. Other candidates incorrectly drew hybrid orbitals.
The orbital diagram in (ii) also proved to be quite a good discriminating question. Many candidates failed to realise that the electrons are removed from the 4s level before the 3d for a first-row transition metal ion. In addition, a significant number of candidates showed poor understanding of Hund‟s Rule of Maximum Multiplicity which states that when degenerate orbitals are available, electrons fill the orbitals singly before filling them in pairs. Hence, in many cases incorrect representations were seen for the 3d which involved three pairs of electrons of opposite spin being inserted in three 3d orbitals. Most candidates stated the \({\text{C}}{{\text{o}}^{2 + }}\) ion, though a common incorrect answer was \({\text{C}}{{\text{o}}^{4 + }}\). Part (iv) involved candidates having to explain why the complex \({\text{[Co(N}}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{6}}}{\text{]C}}{{\text{l}}_{\text{3}}}\) is coloured. This question was asked a number of times in previous examinations and previously was typically really very poorly answered. In N12, the explanations certainly were better though some candidates mixed up the principles of the line emission spectrum of hydrogen with the d to d transitions involved in the explanation of colour pertaining to a transition metal complex.
The Born-Haber cycle for MgO under standard conditions is shown below.
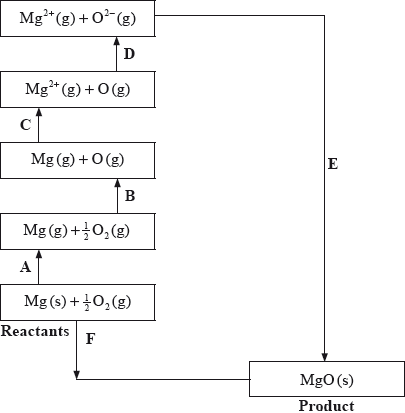
The values are shown in the table below.
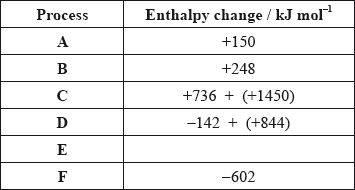
Identify the processes represented by A, B and D in the cycle.
Define the enthalpy change, F.
Determine the value of the enthalpy change, E.
Define the enthalpy change C for the first value. Explain why the second value is significantly larger than the first.
The inter-ionic distance between the ions in NaF is very similar to that between the ions in MgO. Suggest with a reason, which compound has the higher lattice enthalpy value.
The standard enthalpy change of three combustion reactions is given below in kJ.
\[\begin{array}{*{20}{l}} {{\text{2}}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{6}}}{\text{(g)}} + {\text{7}}{{\text{O}}_{\text{2}}}{\text{(g)}} \to {\text{4C}}{{\text{O}}_{\text{2}}}{\text{(g)}} + {\text{6}}{{\text{H}}_{\text{2}}}{\text{O(l)}}}&{\Delta {H^\Theta } = - 3120} \\ {{\text{2}}{{\text{H}}_2}({\text{g)}} + {{\text{O}}_2}{\text{(g)}} \to {\text{2}}{{\text{H}}_2}{\text{O(l)}}}&{\Delta {H^\Theta } = - {\text{572}}} \\ {{{\text{C}}_2}{{\text{H}}_4}({\text{g)}} + {\text{3}}{{\text{O}}_2}{\text{(g)}} \to {\text{2C}}{{\text{O}}_2}{\text{(g)}} + 2{{\text{H}}_2}{\text{O(l)}}}&{\Delta {H^\Theta } = - {\text{1411}}} \end{array}\]
Based on the above information, calculate the standard change in enthalpy, \(\Delta {H^\Theta }\), for the following reaction.
\[{{\text{C}}_2}{{\text{H}}_6}({\text{g)}} \to {{\text{C}}_2}{{\text{H}}_4}({\text{g)}} + {{\text{H}}_2}{\text{(g)}}\]
Predict, stating a reason, whether the sign of \({\Delta {S^\Theta }}\) for the above reaction would be positive or negative.
Discuss why the above reaction is non-spontaneous at low temperature but becomes spontaneous at high temperatures.
Using bond enthalpy values, calculate \(\Delta {H^\Theta }\) for the following reaction.
\[{{\text{C}}_2}{{\text{H}}_6}({\text{g)}} \to {{\text{C}}_2}{{\text{H}}_4}({\text{g)}} + {{\text{H}}_2}{\text{(g)}}\]
Suggest with a reason, why the values obtained in parts (b) (i) and (b) (iv) are different.
Markscheme
A: sublimation/atomization;
B: atomization/half dissociation enthalpy;
D: (sum of 1st and 2nd) electron affinity;
Do not accept vaporization for A and B.
Accept \(\Delta {H_{AT}}\)\( \cdot \) or \(\Delta {H_{EA}}\).
enthalpy change when one mole of the compound is formed from its elements (in their standard states);
under standard conditions / 25 °C/298 K and 1 atm/\({\text{101.3 kPa/1.01}} \times {\text{105 Pa}}\);
\( - 602 = 150 + 248 + 2186 + 702 + E\);
\( - {\text{3888 (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Do not allow 3889 (given in data booklet).
Allow 3888 (i.e no minus sign).
Award [2] for the correct final answer.
energy required to remove one electron;
from an atom in its gaseous state;
electron removed from a positive ion;
decrease in electron-electron repulsion / increase in nucleus-electron attraction;
MgO;
double ionic charge / both ions carry +2 and –2 charge/greater charge compared to +1 and –1;
\[\begin{array}{*{20}{l}} {\left( {{{\text{C}}_2}{{\text{H}}_6}{\text{(g)}} + {\text{3}}\frac{1}{2}{{\text{O}}_2}{\text{(g)}} \to {\text{2C}}{{\text{O}}_2}{\text{(g)}} + {\text{3}}{{\text{H}}_2}{\text{O(l)}}} \right)}&{\Delta {H^\Theta } = - {\text{1560;}}} \\ {\left( {{{\text{H}}_2}{\text{O(l)}} \to {{\text{H}}_2}{\text{(g)}} + \frac{1}{2}{{\text{O}}_2}{\text{(g)}}} \right)}&{\Delta {H^\Theta } = + {\text{286;}}} \\ {\left( {{\text{2C}}{{\text{O}}_2}{\text{(g)}} + {\text{2}}{{\text{H}}_2}{\text{O(l)}} \to {{\text{C}}_2}{{\text{H}}_4}{\text{(g)}} + {\text{3}}{{\text{O}}_2}{\text{(g)}}} \right)}&{\Delta {H^\Theta } = + {\text{1411;}}} \\ {\left( {{{\text{C}}_2}{{\text{H}}_6}{\text{(g)}} \to {{\text{C}}_2}{{\text{H}}_4}{\text{(g)}} + {{\text{H}}_2}{\text{(g)}}} \right)}&{\Delta {H^\Theta } = + {\text{137 (kJ);}}} \end{array}\]
Allow other correct methods.
Award [2] for –137.
Allow ECF for the final marking point.
positive;
increase in number of moles of gas;
at low temperature, \({\Delta {H^\Theta }}\) is positive and \({\Delta G}\) is positive;
at high temperature, factor \({\text{T}}\Delta {S^\Theta }\) predominates and \({\Delta G}\) is negative;
Bonds broken (1C–C, 6C–H, or 1C–C, 2C–H) = 2825/1173;
Bonds made (1C=C, 1H–H, 4C–H) = 2700/1048;
+125 (kJ);
Allow 125 but not –125 (kJ ) for the final mark.
Award [3] for the correct final answer.
bond enthalpy values are average values;
Examiners report
This was the second most popular question and in general candidates demonstrated a good understanding of the Born Haber cycle. Some candidates identified the process A as vaporization instead of atomization.
Most candidates correctly stated the definition of enthalpy change of formation although some omitted to specify the standard conditions.
The majority of candidates correctly calculated the lattice enthalpy value.
The definition of the first ionization energy was stated correctly by most candidates but in a few cases the term gaseous state was missing.
The compound with higher lattice enthalpy was correctly identified including the reason.
The majority of candidates manipulated the thermo-chemical equations and calculated the correct answer of +137 kJ although some reversed the sign.
The explanation for why the reaction was non-spontaneous at low temperature but became spontaneous at high temperature was not always precise and deprived many candidates of at least one mark.
The bond enthalpy calculation had the usual mistakes of using the wrong value from the data booklet, bond making minus bond breaking and –125 kJ instead of +125 kJ.
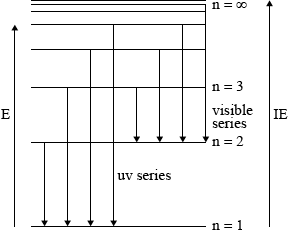
On the above diagram, draw the line that corresponds to the first ionization energy of hydrogen and explain your reasoning.
Markscheme
for showing the energy to remove electron from \({\text{n}} = 1\) to \({\text{n}} = \infty \) on the above diagram;
to ionize an element, electron must be removed from the atom/no longer under influence of nucleus/removed beyond \({\text{n}} = \infty \) / OWTTE;
Examiners report
Candidates in some schools, however, appeared not to have encountered these ideas at all. Common errors were to label the first energy level as\({\text{n}} = 0\) rather than\({\text{n}} = 1\) and to only include one transition for each series. Sometimes the arrows showing the transitions were shown from the bottom up.
While more candidates managed to obtain at least a mark with regard to the first ionization energy of hydrogen, it was less common to find the correct graphical representation of the first IE with diagrams which often were unrelated.
Hydrogen peroxide decomposes according to the equation below.
\({\text{2}}{{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}{\text{(aq)}} \to {\text{2}}{{\text{H}}_{\text{2}}}{\text{O(l)}} + {{\text{O}}_{\text{2}}}{\text{(g)}}\)
The rate of the decomposition can be monitored by measuring the volume of oxygen gas released. The graph shows the results obtained when a solution of hydrogen peroxide decomposed in the presence of a CuO catalyst.
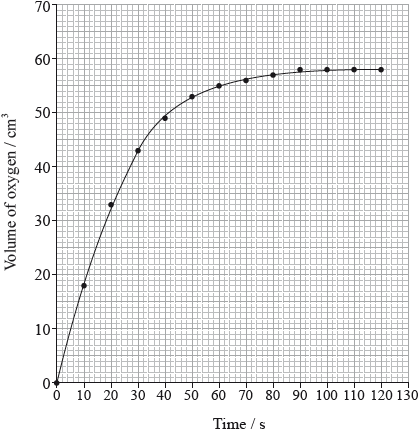
Outline how the initial rate of reaction can be found from the graph.
Explain how and why the rate of reaction changes with time.
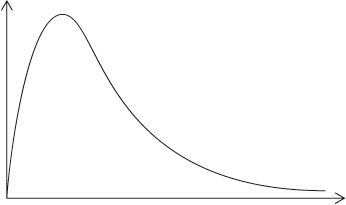
A Maxwell-Boltzmann energy distribution curve is drawn below. Label both axes and explain, by annotating the graph, how catalysts increase the rate of reaction.
(i) In some reactions, increasing the concentration of a reactant does not increase the rate of reaction. Describe how this may occur.
(ii) Consider the reaction
\[{\text{2A}} + {\text{B}} \to {\text{C}} + {\text{D}}\]
The reaction is first order with respect to A, and zero order with respect to B. Deduce the rate expression for this reaction.
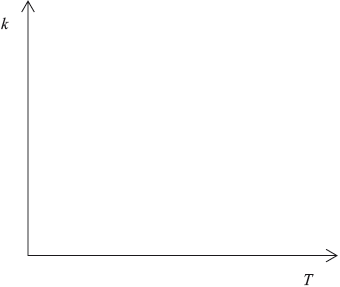
Sketch a graph of rate constant \((k)\) versus temperature.
Hydrochloric acid neutralizes sodium hydroxide, forming sodium chloride and water.
\({\text{NaOH(aq)}} + {\text{HCl(aq)}} \to {\text{NaCl(aq)}} + {{\text{H}}_{\text{2}}}{\text{O(l)}}\) \(\Delta {H^\Theta } = - 57.9{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\)
(i) Define standard enthalpy change of reaction, \(\Delta {H^\Theta }\).
(ii) Determine the amount of energy released, in kJ, when \({\text{50.0 c}}{{\text{m}}^{\text{3}}}\) of \({\text{1.00 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) sodium hydroxide solution reacts with \({\text{50.0 c}}{{\text{m}}^{\text{3}}}\) of \({\text{1.00 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) hydrochloric acid solution.
(iii) In an experiment, 2.50 g of solid sodium hydroxide was dissolved in \({\text{50.0 c}}{{\text{m}}^{\text{3}}}\) of water. The temperature rose by 13.3 °C. Calculate the standard enthalpy change, in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), for dissolving one mole of solid sodium hydroxide in water.
\[{\text{NaOH(s)}} \to {\text{NaOH(aq)}}\]
(iv) Using relevant data from previous question parts, determine \(\Delta {H^\Theta }\), in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), for the reaction of solid sodium hydroxide with hydrochloric acid.
\[{\text{NaOH(s)}} + {\text{HCl(aq)}} \to {\text{NaCl(aq)}} + {{\text{H}}_{\text{2}}}{\text{O(l)}}\]
(i) Zinc is found in the d-block of the periodic table. Explain why it is not considered a transition metal.
(ii) Explain why \({\text{F}}{{\text{e}}^{3 + }}\) is a more stable ion than \({\text{F}}{{\text{e}}^{2 + }}\) by reference to their electron configurations.
Markscheme
(draw a) tangent to the curve at origin/time = 0/start of reaction;
(calculate) the gradient/slope (of the tangent);
rate decreases (with time);
concentration/number of (reactant) molecules per unit volume decreases (with time);
Do not accept “number of molecules decreases” or “amount of reactant decreases”.
collisions (between reactant molecules/reactant and catalyst) become less frequent;
Do not accept “fewer collisions” without reference to frequency (eg, no. collisions per second).
y-axis: probability / fraction of molecules/particles / probability density
Allow “number of particles/molecules” on y-axis.
and
x-axis: (kinetic) energy;
Accept “speed/velocity” on x-axis.
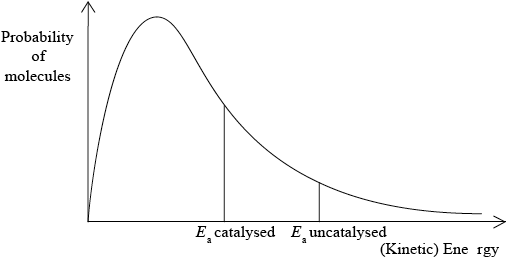
correct relative position of \({E_{\text{a}}}\) catalysed and \({E_{\text{a}}}\) uncatalysed;
more/greater proportion of molecules/collisions have the lower/required/catalysed \({E_{\text{a}}}\) (and can react upon collision);
M3 can be scored by stating or shading and annotating the graph.
Accept “a greater number/proportion of successful collisions as catalyst reduces \({E_a}\)”.
(i) reactant not involved in (or before) the slowest/rate-determining step/RDS;
reactant is in (large) excess;
(ii) \({\text{(rate}} = {\text{) }}k{\text{[A]}}\);
Accept rate = k[A]1[B]0.
curve with a positive slope curving upwards;
Do not penalize if curve passes through the origin.
(i) heat transferred/absorbed/released/enthalpy/potential energy change when 1 mol/molar amounts of reactant(s) react (to form products) / OWTTE;
under standard conditions / at a pressure 100 kPa/101.3 kPa/1 atm and temperature 298 K/25 °C;
Award [2] for difference between standard enthalpies of products and standard enthalpies of reactants / \({H^\Theta }\) (products) – \({H^\Theta }\) (reactants).
Award [2] for difference between standard enthalpies of formation of products and standard enthalpies of formation of reactants / \(\Sigma \Delta H_f^\Theta \) (products) – \(\Sigma \Delta H_f^\Theta \) (reactants).
(ii) \((1.00 \times 0.0500 = ){\text{ }}0.0500{\text{ (mol)}}\);
\((0.0500 \times 57.9 = ){\text{ }}2.90{\text{ (kJ)}}\);
Ignore any negative sign.
Award [2] for correct final answer.
Award [1 max] for 2900 J.
(iii) \(\left( {\frac{{2.50}}{{40.00}} = } \right){\text{ }}0.0625{\text{ (mol NaOH)}}\);
\(0.0500 \times 4.18 \times 13.3 = 2.78{\text{ (kJ)}}/50.0 \times 4.18 \times 13.3 = 2780{\text{ (J)}}\);
\(\left( {\frac{{2.78}}{{0.0625}}} \right) = - 44.5{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}})\);
Award [3] for correct final answer.
Negative sign is necessary for M3.
Award M2 and M3 if is used to obtain an enthalpy change of –46.7 (kJ mol–1).
(iv) \( - 44.5 - 57.9\) / correct Hess’s Law cycle (as below) / correct manipulation of equations;
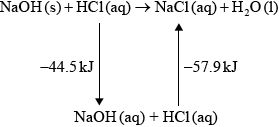
\( - 102.4{\text{ kJ}}\);
Award [2] for correct final answer.
(i) zinc (only) forms the ion \({\text{Z}}{{\text{n}}^{2 + }}\) / has the oxidation state \( + 2\);
Allow forms only one ion / has only one oxidation state.
has full d-subshell/orbitals / does not have a partially filled d-subshell/orbitals (needed to exhibit transition metal properties);
(ii) \({\text{F}}{{\text{e}}^{2 + }}{\text{: 1}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{s}}^{\text{2}}}{\text{3}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{d}}^{\text{6}}}/{\text{[Ar] 3}}{{\text{d}}^{\text{6}}}\) and \({\text{F}}{{\text{e}}^{3 + }}{\text{: 1}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{s}}^{\text{2}}}{\text{3}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{d}}^{\text{5}}}/{\text{[Ar] 3}}{{\text{d}}^{\text{5}}}\);
half-full sub-level/3d5 has extra stability;
less repulsion between electrons / electrons singly occupy orbitals / electrons do not have to pair with other electrons;
Accept converse points for Fe2+.
Examiners report
Most candidates related the rate of reaction to the gradient of the curve, but only a few suggested drawing a tangent at \(t = 0\).
Answers were often disappointing and only a few candidates gained full marks.
Candidates often talked about the number of reactant molecules decreasing but neglected to relate this to a lower concentration. Also some candidates still fail to highlight frequency rather than the number of collisions.
Well answered by more than half of the candidates. The labelling of the axes was a challenge for some candidates. The annotation of the diagram with the energy of activation with and without a catalyst was mostly correct, though some weaker students confused it with the effect of temperature and constructed a second curve. Some candidates could not offer an explanation for the third mark.
(i) Only a few candidates scored this mark. Many candidates stated that a reactant concentration having no effect indicated that the reaction that was zero order in that species, rather than describing the underlying mechanistic reason for the zero order dependence.
(ii) More than half of the candidates could construct a correct rate expression from information about the order of the reactants.
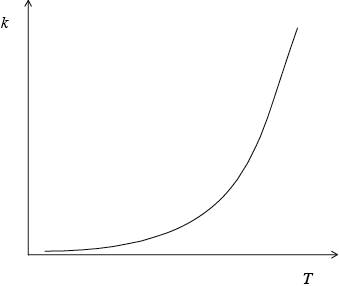
A number of candidates gave a linear relationship, rather than an exponential one, between reaction rate and temperature.
(i) Defining the standard enthalpy change of reaction was not well answered.
(ii) More than half of the candidates calculated the amount of energy released correctly.
(iii) Half of the candidates were able to gain the three marks. Many candidates lost the third mark for not quoting the negative sign for the enthalpy change. Quite a few candidates used a wrong value for the mass of water.
(iv) Many good answers. A Hess’s Law cycle wasn’t often seen. Quite a few candidates scored through ECF from (iii).
(i) Most candidates knew that zinc has a full 3d sub-shell but almost all missed out on the second mark about only having one possible oxidation state in its compounds.
(ii) This was a challenging question for many candidates. A large number of candidates did not give the correct electron configurations for the ions, and only few mentioned the stability of the half-full d-sub-shell. Very few scored the third mark.
Calcium carbide, CaC2, is an ionic solid.
Describe the nature of ionic bonding.
Describe how the relative atomic mass of a sample of calcium could be determined from its mass spectrum.
When calcium compounds are introduced into a gas flame a red colour is seen; sodium compounds give a yellow flame. Outline the source of the colours and why they are different.
Suggest two reasons why solid calcium has a greater density than solid potassium.
Outline why solid calcium is a good conductor of electricity.
Sketch a graph of the first six ionization energies of calcium.
Calcium carbide reacts with water to form ethyne and calcium hydroxide.
CaC2(s) + H2O(l) → C2H2(g) + Ca(OH)2(aq)
Estimate the pH of the resultant solution.
Describe how sigma (σ) and pi (\(\pi \)) bonds are formed.
Deduce the number of σ and \(\pi \) bonds in a molecule of ethyne.
Markscheme
electrostatic attraction AND oppositely charged ions
[1 mark]
multiply relative intensity by «m/z» value of isotope
OR
find the frequency of each isotope
sum of the values of products/multiplication «from each isotope»
OR
find/calculate the weighted average
Award [1 max] for stating “m/z values of isotopes AND relative abundance/intensity” but not stating these need to be multiplied.
[2 marks]
«promoted» electrons fall back to lower energy level
energy difference between levels is different
Accept “Na and Ca have different nuclear charge” for M2.
[2 marks]
Any two of:
stronger metallic bonding
smaller ionic/atomic radius
two electrons per atom are delocalized
OR
greater ionic charge
greater atomic mass
Do not accept just “heavier” or “more massive” without reference to atomic mass.
[2 marks]
delocalized/mobile electrons «free to move»
[1 mark]
general increase
only one discontinuity between “IE2” and “IE3”
[2 marks]
pH > 7
Accept any specific pH value or range of values above 7 and below 14.
[1 mark]
sigma (σ):
overlap «of atomic orbitals» along the axial/internuclear axis
OR
head-on/end-to-end overlap «of atomic orbitals»
pi (\(\pi \)):
overlap «of p-orbitals» above and below the internuclear axis
OR
sideways overlap «of p-orbitals»
Award marks for suitable diagrams.
[2 marks]
sigma (σ): 3
AND
pi (\(\pi \)): 2
[1 mark]
Examiners report
Iron has three main naturally occurring isotopes which can be investigated using a mass spectrometer.
State the full electronic configurations of a Cu atom and a \({\text{C}}{{\text{u}}^ + }\) ion.
Cu:
\({\text{C}}{{\text{u}}^ + }\):
Explain the origin of colour in transition metal complexes and use your explanation to suggest why copper(II) sulfate, CuSO4(aq), is blue, but zinc sulfate, ZnSO4(aq), is colourless.
\({\text{C}}{{\text{u}}^{2 + }}{\text{(aq)}}\) reacts with ammonia to form the complex ion \({{\text{[Cu(N}}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{4}}}{\text{]}}^{2 + }}\). Explain this reaction in terms of an acid-base theory, and outline how the bond is formed between \({\text{C}}{{\text{u}}^{2 + }}\) and \({\text{N}}{{\text{H}}_{\text{3}}}\).
Markscheme
Cu:
\({\text{1}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{s}}^{\text{2}}}{\text{3}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{d}}^{{\text{10}}}}{\text{4}}{{\text{s}}^{\text{1}}}\);
\({\text{C}}{{\text{u}}^ + }\):
\({\text{1}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{s}}^{\text{2}}}{\text{3}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{d}}^{{\text{10}}}}\);
Ignore relative order of 3d and 4s.
Penalize only once if noble gas core is given.
d orbitals are split (into two sets of different energies);
frequencies of (visible) light absorbed by electrons moving from lower to higher d levels;
colour due to remaining frequencies/complementary colour transmitted;
\({\text{C}}{{\text{u}}^{2 + }}\) has unpaired electrons/partially filled d sub-level;
\({\text{Z}}{{\text{n}}^{2 + }}\) has filled d sub-shell;
electronic transitions/d-d transitions possible for \({\text{C}}{{\text{u}}^{2 + }}\) / no electronic/d-d transitions possible for \({\text{Z}}{{\text{n}}^{2 + }}\);
Allow wavelength as well as frequency.
\({\text{N}}{{\text{H}}_{\text{3}}}\): Lewis base / \({\text{C}}{{\text{u}}^{2 + }}\): Lewis acid;
each \({\text{N}}{{\text{H}}_{\text{3}}}\)/ligand donates an electron pair (to \({\text{C}}{{\text{u}}^{2 + }}\));
\({\text{N}}{{\text{H}}_{\text{3}}}\) replace \({{\text{H}}_2}{\text{O}}\) ligands around \({\text{C}}{{\text{u}}^{2 + }}\) ion/around central ion;
forming coordinate (covalent)/dative covalent bond;
Examiners report
Many candidates identified the electronic configuration of Cu as an exception but the 3d electron was often removed in forming the ion instead of the 4s.
Precision of language proved to be an issue in (e) with some candidates referring to Cu and Zn and not their ions and some students explained the colour as a result of “reflection” or “emission”.
In (f), many candidates mentioned proton donors and proton acceptors and made no reference to Lewis theory.
The oxides and chlorides of period 3 elements exhibit periodicity.
Chlorine gas, \({\text{C}}{{\text{l}}_{\text{2}}}{\text{(g)}}\), is bubbled through separate solutions of aqueous bromine, \({\text{B}}{{\text{r}}_{\text{2}}}{\text{(aq)}}\), and potassium bromide, \({\text{KBr(aq)}}\).
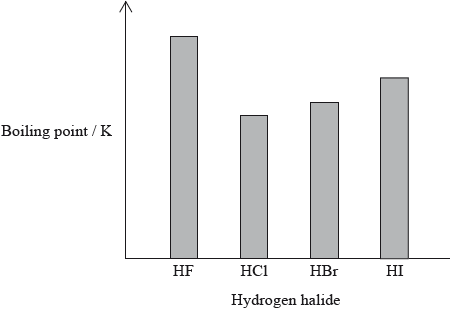
The hydrogen halides do not show perfect periodicity. A bar chart of boiling points shows that the boiling point of hydrogen fluoride, HF, is much higher than periodic trends would indicate.
Transition metals form complex ions which are usually coloured.
(i) State the changes in the acid-base nature of the oxides across period 3 (from \({\text{N}}{{\text{a}}_2}{\text{O}}\) to \({\text{C}}{{\text{l}}_{\text{2}}}{{\text{O}}_{\text{7}}}\)), including equations for the reactions of \({\text{N}}{{\text{a}}_2}{\text{O}}\) and \({\text{S}}{{\text{O}}_{\text{3}}}\) with water.
(ii) State whether or not molten aluminium chloride, \({\text{A}}{{\text{l}}_{\text{2}}}{\text{C}}{{\text{l}}_{\text{6}}}\), and molten aluminium oxide, \({\text{A}}{{\text{l}}_{\text{2}}}{{\text{O}}_{\text{3}}}\), conduct electricity. Explain this behaviour in terms of the structure and bonding of the two compounds.
(iii) State the equation for the reaction of \({\text{C}}{{\text{l}}_{\text{2}}}\) with water.
(i) Predict any changes that may be observed in each case.
\({\text{B}}{{\text{r}}_{\text{2}}}{\text{(aq)}}\):
\({\text{KBr(aq)}}\):
(ii) State the half-equations for the reactions that occur.
(i) Explain why the boiling point of HF is much higher than the boiling points of the other hydrogen halides.
(ii) Explain the trend in the boiling points of HCl, HBr and HI.
State the full electron configurations of Cr and \({\text{C}}{{\text{r}}^{3 + }}\).
Cr:
\({\text{C}}{{\text{r}}^{3 + }}\):
\({\text{C}}{{\text{r}}^{3 + }}\) ions and water molecules bond together to form the complex ion \({{\text{[Cr(}}{{\text{H}}_{\text{2}}}{\text{O}}{{\text{)}}_{\text{6}}}{\text{]}}^{3 + }}\).
Describe how the water acts and how it forms the bond, identifying the acid-base character of the reaction.
Explain why the \({{\text{[Cr(}}{{\text{H}}_{\text{2}}}{\text{O}}{{\text{)}}_{\text{6}}}{\text{]}}^{3 + }}\) ion is coloured.
Outline, including a relevant equation, whether the \({{\text{[Cr(}}{{\text{H}}_{\text{2}}}{\text{O}}{{\text{)}}_{\text{6}}}{\text{]}}^{3 + }}\) ion is acidic, basic or neutral.
Explain how the number of electrons in the outer main energy level of phosphorus, P, can be determined using the data of successive ionization energies.
Markscheme
(i) basic to acidic;
\({\text{N}}{{\text{a}}_{\text{2}}}{\text{O(s)}} + {{\text{H}}_{\text{2}}}{\text{O(l)}} \to {\text{2NaOH(aq)}}\);
\({\text{S}}{{\text{O}}_3}{\text{(g)}} + {{\text{H}}_2}{\text{O(l)}} \to {{\text{H}}_2}{\text{S}}{{\text{O}}_4}{\text{(aq)}}\);
Ignore state symbols.
(ii) molten \({\text{A}}{{\text{l}}_2}{\text{C}}{{\text{l}}_6}\) does not conduct electricity and molten \({\text{A}}{{\text{l}}_{\text{2}}}{{\text{O}}_{\text{3}}}\) does;
\({\text{A}}{{\text{l}}_2}{\text{C}}{{\text{l}}_6}\) is a covalent molecule and has no free charged particles to conduct electricity;
\({\text{A}}{{\text{l}}_2}{{\text{O}}_3}\) is ionic/has ions which are free to move when molten;
(iii) \({\text{C}}{{\text{l}}_2}{\text{(g)}} + {{\text{H}}_2}{\text{O(l)}} \rightleftharpoons {\text{HCl(aq)}} + {\text{HClO(aq)}}\);
Ignore state symbols.
Allow \( \to \).
(i) \({\text{B}}{{\text{r}}_2}{\text{(aq)}}\): no change;
\({\text{KBr(aq)}}\): colour change / from colourless to red/yellow/orange/brown;
(ii) \({\text{2B}}{{\text{r}}^ - }{\text{(aq)}} \to {\text{B}}{{\text{r}}_2}{\text{(aq)}} + {\text{2}}{{\text{e}}^ - }\);
\({\text{C}}{{\text{l}}_2}{\text{(g)}} + {\text{2}}{{\text{e}}^ - } \to {\text{2C}}{{\text{l}}^ - }{\text{(aq)}}\);
Ignore state symbols.
Accept e instead of e–.
(i) HF has hydrogen bonds (between molecules);
(ii) strength of van der Waals’/London/dispersion forces increases;
as mass/size/number of electrons of halogen atom/molecule increases;
Cr: \({\text{1}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{s}}^{\text{2}}}{\text{3}}{{\text{p}}^{\text{6}}}{\text{4}}{{\text{s}}^{\text{1}}}{\text{3}}{{\text{d}}^{\text{5}}}/{\text{1}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{s}}^{\text{2}}}{\text{3}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{d}}^{\text{5}}}{\text{4}}{{\text{s}}^{\text{1}}}\);
Cr3+: \({\text{1}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{s}}^{\text{2}}}{\text{3}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{d}}^{\text{3}}}\);
\({{\text{H}}_2}{\text{O}}\) is a ligand / has lone (electron) pair;
forms dative (covalent)/coordinate bond / donates a lone (electron) pair ;
ligand is Lewis base / \({\text{C}}{{\text{r}}^{3 + }}\) is Lewis acid;
\({\text{C}}{{\text{r}}^{3 + }}\) has partially filled d orbitals;
d orbitals split into two levels / three lower energy and two higher energy levels;
energy difference is in visible part of spectrum;
electrons absorb visible light / one colour/frequency/wavelength;
electron transitions occur from lower to higher energy level within d sub-level;
complementary colour/colour not absorbed is seen;
acidic because \({{\text{[Cr(}}{{\text{H}}_2}{\text{O}}{{\text{)}}_6}{\text{]}}^{3 + }}{\text{(aq)}} \to {{\text{[Cr(}}{{\text{H}}_2}{\text{O}}{{\text{)}}_5}{\text{(OH)]}}^{2 + }}{\text{(aq)}} + {{\text{H}}^ + }{\text{(aq)}}\);
Allow answers with further equations.
Accept any other valid equations.
Ignore state symbols.
successive ionization energy values increase with removal of each electron;
large increase in ionization energy when sixth electron is removed;
as electron is one energy level/shell closer to the nucleus;
Accept a suitably annotated diagram.
Examiners report
There appeared to be some significant gaps in knowledge within this question, the various parts either scored very well or not at all.
In a(ii) there was a poor understanding of the nature of bonding in aluminium chloride and aluminium oxide. Candidates are still confusing electrical conductivity in compounds with that in metals, and often refer to the inability to conduct being down to a lack of mobile electrons in compounds.
Balancing equations, both full, as in parts a(i) and a(iii), and half equations as in b(ii), showed poor knowledge both of the reactants and products and in the ability to balance them in both atoms and charge. It should be expected that higher level candidates would be comfortable with these processes. The ability to deduce and predict what they would see during a reaction is a skill required of all chemists, it was missing in the attempts to answer b(ii). Parts c and d(i), (ii) and (iii) showed good knowledge, but in part d(iv) the understanding of the acid nature of some d block complex ions was lacking. Part e was rarely given credit, as many appeared to misread the question, and discussed the changes in first ionisation energies across Period 3.
There appeared to be some significant gaps in knowledge within this question, the various parts either scored very well or not at all.
In a(ii) there was a poor understanding of the nature of bonding in aluminium chloride and aluminium oxide. Candidates are still confusing electrical conductivity in compounds with that in metals, and often refer to the inability to conduct being down to a lack of mobile electrons in compounds.
Balancing equation,s both full, as in parts a(i) and a(iii), and half equations as in b(ii), showed poor knowledge both of the reactants and products and in the ability to balance them in both atoms and charge. It should be expected that higher level candidates would be comfortable with these processes. The ability to deduce and predict what they would see during a reaction is a skill required of all chemists, it was missing in the attempts to answer b(ii). Parts c and d(i), ii) and iii) showed good knowledge, but in part d(iv) the understanding of the acid nature of some d block complex ions was lacking. Part e was rarely given credit, as many appeared to misread the question, and discussed the changes in first ionisation energies across Period 3.
There appeared to be some significant gaps in knowledge within this question, the various parts either scored very well or not at all.
In a(ii) there was a poor understanding of the nature of bonding in aluminium chloride and aluminium oxide. Candidates are still confusing electrical conductivity in compounds with that in metals, and often refer to the inability to conduct being down to a lack of mobile electrons in compounds.
Balancing equations, both full, as in parts a(i) and a(iii), and half equations as in b(ii), showed poor knowledge both of the reactants and products and in the ability to balance them in both atoms and charge. It should be expected that higher level candidates would be comfortable with these processes. The ability to deduce and predict what they would see during a reaction is a skill required of all chemists, it was missing in the attempts to answer b(ii). Parts c and d(i), ii) and iii) showed good knowledge, but in part d(iv) the understanding of the acid nature of some d block complex ions was lacking. Part e was rarely given credit, as many appeared to misread the question, and discussed the changes in first ionisation energies across Period 3.
There appeared to be some significant gaps in knowledge within this question, the various parts either scored very well or not at all.
In a(ii) there was a poor understanding of the nature of bonding in aluminium chloride and aluminium oxide. Candidates are still confusing electrical conductivity in compounds with that in metals, and often refer to the inability to conduct being down to a lack of mobile electrons in compounds.
Balancing equations, both full, as in parts a(i) and a(iii), and half equations as in b(ii), showed poor knowledge both of the reactants and products and in the ability to balance them in both atoms and charge. It should be expected that higher level candidates would be comfortable with these processes. The ability to deduce and predict what they would see during a reaction is a skill required of all chemists, it was missing in the attempts to answer b(ii). Parts c and d(i), ii) and iii) showed good knowledge, but in part d(iv) the understanding of the acid nature of some d block complex ions was lacking. Part e was rarely given credit, as many appeared to misread the question, and discussed the changes in first ionisation energies across Period 3.
There appeared to be some significant gaps in knowledge within this question, the various parts either scored very well or not at all.
In a(ii) there was a poor understanding of the nature of bonding in aluminium chloride and aluminium oxide. Candidates are still confusing electrical conductivity in compounds with that in metals, and often refer to the inability to conduct being down to a lack of mobile electrons in compounds.
Balancing equations, both full, as in parts a(i) and a(iii), and half equations as in b(ii), showed poor knowledge both of the reactants and products and in the ability to balance them in both atoms and charge. It should be expected that higher level candidates would be comfortable with these processes. The ability to deduce and predict what they would see during a reaction is a skill required of all chemists, it was missing in the attempts to answer b(ii). Parts c and d(i), ii) and iii) showed good knowledge, but in part d(iv) the understanding of the acid nature of some d block complex ions was lacking. Part e was rarely given credit, as many appeared to misread the question, and discussed the changes in first ionisation energies across Period 3.
There appeared to be some significant gaps in knowledge within this question, the various parts either scored very well or not at all.
In a(ii) there was a poor understanding of the nature of bonding in aluminium chloride and aluminium oxide. Candidates are still confusing electrical conductivity in compounds with that in metals, and often refer to the inability to conduct being down to a lack of mobile electrons in compounds.
Balancing equations, both full, as in parts a(i) and a(iii), and half equations as in b(ii), showed poor knowledge both of the reactants and products and in the ability to balance them in both atoms and charge. It should be expected that higher level candidates would be comfortable with these processes. The ability to deduce and predict what they would see during a reaction is a skill required of all chemists, it was missing in the attempts to answer b(ii). Parts c and d(i), ii) and iii) showed good knowledge, but in part d(iv) the understanding of the acid nature of some d block complex ions was lacking. Part e was rarely given credit, as many appeared to misread the question, and discussed the changes in first ionisation energies across Period 3.
There appeared to be some significant gaps in knowledge within this question, the various parts either scored very well or not at all.
In a(ii) there was a poor understanding of the nature of bonding in aluminium chloride and aluminium oxide. Candidates are still confusing electrical conductivity in compounds with that in metals, and often refer to the inability to conduct being down to a lack of mobile electrons in compounds.
Balancing equations, both full, as in parts a(i) and a(iii), and half equations as in b(ii), showed poor knowledge both of the reactants and products and in the ability to balance them in both atoms and charge. It should be expected that higher level candidates would be comfortable with these processes. The ability to deduce and predict what they would see during a reaction is a skill required of all chemists, it was missing in the attempts to answer b(ii). Parts c and d(i), ii) and iii) showed good knowledge, but in part d(iv) the understanding of the acid nature of some d block complex ions was lacking. Part e was rarely given credit, as many appeared to misread the question, and discussed the changes in first ionisation energies across Period 3.
There appeared to be some significant gaps in knowledge within this question, the various parts either scored very well or not at all.
In a(ii) there was a poor understanding of the nature of bonding in aluminium chloride and aluminium oxide. Candidates are still confusing electrical conductivity in compounds with that in metals, and often refer to the inability to conduct being down to a lack of mobile electrons in compounds.
Balancing equations, both full, as in parts a(i) and a(iii), and half equations as in b(ii) showed poor knowledge both of the reactants and products and in the ability to balance them in both atoms and charge. It should be expected that higher level candidates would be comfortable with these processes. The ability to deduce and predict what they would see during a reaction is a skill required of all chemists, it was missing in the attempts to answer b(ii). Parts c and d(i), ii) and iii) showed good knowledge, but in part d(iv) the understanding of the acid nature of some d block complex ions was lacking. Part e was rarely given credit, as many appeared to misread the question, and discussed the changes in first ionisation energies across Period 3.
Magnesium is the eighth most abundant element in the earth’s crust. The successive ionization energies of the element are shown below.
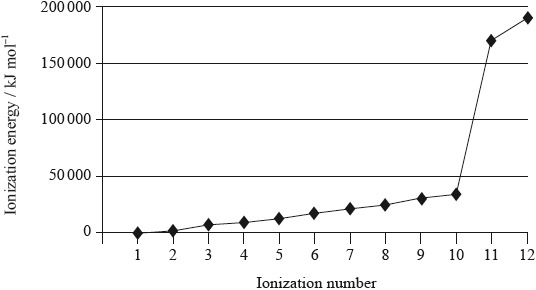
Magnesium can be produced from the electrolysis of molten magnesium chloride, MgCl2.
The lattice enthalpy of magnesium chloride can be calculated from the Born-Haber cycle shown below.
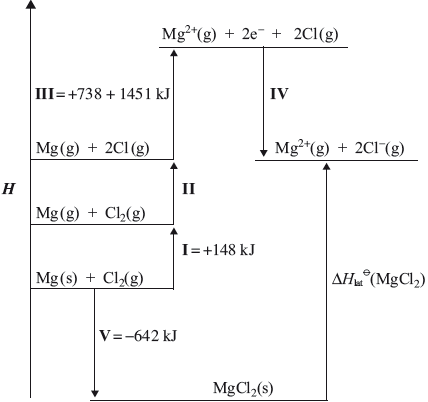
(i) Define the term first ionization energy and state the equation for the first ionization of magnesium.
(ii) Explain the general increase in successive ionization energies of the element.
(iii) Explain the large increase between the tenth and eleventh ionization energies.
(i) Explain how molten magnesium chloride conducts an electric current.
(ii) Identify the electrode where oxidation occurs during electrolysis of molten magnesium chloride and state an equation for the half-reaction.
(iii) Explain why magnesium is not formed during the electrolysis of aqueous magnesium chloride solution.
(i) Identify the enthalpy changes labelled by I and V in the cycle.
(ii) Use the ionization energies given in the cycle above and further data from the Data Booklet to calculate a value for the lattice enthalpy of magnesium chloride.
(iii) The theoretically calculated value for the lattice enthalpy of magnesium chloride is +2326 kJ. Explain the difference between the theoretically calculated value and the experimental value.
(iv) The experimental lattice enthalpy of magnesium oxide is given in Table 13 of the Data Booklet. Explain why magnesium oxide has a higher lattice enthalpy than magnesium chloride.
(i) State whether aqueous solutions of magnesium oxide and magnesium chloride are acidic, alkaline or neutral.
(ii) State an equation for the reaction between magnesium oxide and water.
Markscheme
(i) energy (per mole) needed to remove one/first/most loosely bound electron from a (neutral) atom;
in the gaseous state;
\({\text{Mg(g)}} \to {\text{M}}{{\text{g}}^ + }{\text{(g)}} + {{\text{e}}^ - }\);
Gaseous state symbols needed.
Accept e instead of e–.
Only penalize omission of gas phase once in either the second marking point or the third marking point.
(ii) successive electrons (are more difficult to remove because each is) taken from more positively charged ion/ OWTTE;
increased electrostatic attraction;
(iii) 10th electron comes from 2nd energy level/\(n = 2\) and 11th electron comes from 1st first energy level/\(n = 1\) / OWTTE;
electron in 1st energy level closer to nucleus;
electron in 1st energy level not shielded by inner electrons / exposed to greater effective nuclear charge;
(i) contains ions which are free to move (only) in molten state;
\({\text{M}}{{\text{g}}^{2 + }}\) move to cathode/negative electrode and \({\text{C}}{{\text{l}}^ - }\) move to anode/positive electrode / OWTTE;
(ii) anode/positive electrode;
\({\text{2C}}{{\text{l}}^ - } \to {\text{C}}{{\text{l}}_2} + {\text{2}}{{\text{e}}^ - }/{\text{C}}{{\text{l}}^ - } \to \frac{1}{2}{\text{C}}{{\text{l}}_2}+{{\text{e}}^ - }\);
Accept e instead of e–.
Do not accept \(C{l^ - } \to Cl + {e^ - }\).
Ignore state symbols.
(iii) magnesium has large negative electrode potential / \({E^\Theta }\);
reduction of \({{\text{H}}_2}{\text{O/}}{{\text{H}}^ + }\) to \({{\text{H}}_2}\) has less negative electrode potential;
\({\text{M}}{{\text{g}}^{2 + }}\) not readily reduced (in comparison to \({{\text{H}}_2}{\text{O}}\));
if formed, magnesium would (immediately) react with water to form \({\text{M}}{{\text{g}}^{2 + }}\);
magnesium more reactive than hydrogen;
Do not accept Mg too reactive.
(i) I:
atomization/sublimation (of Mg) / \(\Delta H_{{\text{atomization}}}^\Theta {\text{(Mg)}}\) / \(\Delta H_{{\text{sublimation}}}^\Theta {\text{(Mg)}}\);
V:
enthalpy change of formation of \({\text{(MgC}}{{\text{l}}_2}{\text{)}}\) / \(\Delta H_{{\text{formation}}}^\Theta {\text{(MgC}}{{\text{l}}_2}{\text{)}}\);
(ii) Energy value for II:
\( + 243\);
Energy value for III:
\(738 + 1451 = 2189\);
Energy value for IV:
\(2( - 349)\);
\(\Delta H_{{\text{lat}}}^\Theta {\text{(MgC}}{{\text{l}}_2}{\text{)}} = 642 + 148 + 243 + 2189 - 2(349) = ( - )2524{\text{ (kJ)}}\);
(iii) theoretical value assumes ionic model;
experimental value greater due to (additional) covalent character;
(iv) oxide greater charge;
oxide smaller radius;
Accept opposite arguments.
(i) \({\text{MgC}}{{\text{l}}_2}\) (weakly) acidic and MgO alkaline;
(ii) \({\text{MgO}} + {{\text{H}}_2}{\text{O}} \to {\text{Mg(OH}}{{\text{)}}_2}\);
Ignore state symbols.
Examiners report
In (a) several candidates failed to mention atoms in their definition of first ionization energy and others neglected to state that the gaseous state is involved. Only the strongest students mentioned the electrostatic nature of the attraction between the nucleus and the electrons in explaining trends in ionisation energies. Several candidates lost a mark in explaining the increase between the tenth and eleventh ionisation energies as their arguments were incomplete, with no reference to the change from\(n = 2\) energy level to the\(n = 1\) level.
In (b) many candidates stated that free electrons rather than ions were responsible for the conductivity of magnesium chloride and others did not refer to the movement of both \({\text{M}}{{\text{g}}^{2 + }}\) and \({\text{C}}{{\text{l}}^ - }\) ions. The anode was generally identified as the electrode where oxidation occurs but some had difficulties giving the balanced equation for the half reaction. Only the strongest candidates were able to explain why magnesium is not formed during the electrolysis of aqueous solutions.
In (c) most candidates were familiar with the enthalpy changes of atomization and formation but some struggled with the Born Haber Cycle. Only the strongest candidates were able to relate differences in experimental and theoretical lattice energies to the covalent character of the solid with a significant number mistakenly giving “heat loss” as the reason for the difference.
In (d) many were able to correctly describe the basic nature of magnesium oxide but the acidity of magnesium chloride was less well known. Some gave hydrogen gas as a product in the reaction between magnesium oxide and water.
Magnesium is a group 2 metal which exists as a number of isotopes and forms many compounds.
Magnesium ions produce no emission or absorption lines in the visible region of the electromagnetic spectrum. Suggest why most magnesium compounds tested in a school laboratory show traces of yellow in the flame.
(i) Explain the convergence of lines in a hydrogen emission spectrum.
(ii) State what can be determined from the frequency of the convergence limit.
Magnesium chloride can be electrolysed.
(i) Deduce the half-equations for the reactions at each electrode when molten magnesium chloride is electrolysed, showing the state symbols of the products. The melting points of magnesium and magnesium chloride are 922K and 987K respectively.
(ii) Identify the type of reaction occurring at the cathode (negative electrode).
(iii) State the products when a very dilute aqueous solution of magnesium chloride is electrolysed.
Standard electrode potentials are measured relative to the standard hydrogen electrode. Describe a standard hydrogen electrode.
A magnesium half-cell, Mg(s)/Mg2+(aq), can be connected to a copper half-cell, Cu(s)/Cu2+(aq).
(i) Formulate an equation for the spontaneous reaction that occurs when the circuit is completed.
(ii) Determine the standard cell potential, in V, for the cell. Refer to section 24 of the data booklet.
(iii) Predict, giving a reason, the change in cell potential when the concentration of copper ions increases.
Markscheme
contamination with sodium/other «compounds»
i
energy levels are closer together at high energy / high frequency / short wavelength
ii
ionisation energy
i)
Anode (positive electrode):
2Cl– → Cl2 (g) + 2e–
Cathode (negative electrode):
Mg2+ + 2e– → Mg (l)
Penalize missing/incorrect state symbols at Cl2 and Mg once only.
Award [1 max] if equations are at wrong electrodes.
Accept Mg (g).
ii)
reduction
iii)
Anode (positive electrode):
oxygen/O2
OR
hydogen ion/proton/H+ AND oxygen/O2
Cathode (negative electrode):
hydrogen/H2
OR
hydroxide «ion»/OH– AND hydrogen/H2
Award [1 max] if correct products given at wrong electrodes.
Any two of:
«inert» Pt electrode
OR
platinum black conductor
1 mol dm–3 H+ (aq)
H2 (g) at 100 kPa
Accept 1 atm H2 (g).
Accept 1 bar H2 (g)
Accept a labelled diagram.
Ignore temperature if it is specified.
i
Mg(s) + Cu2+ (aq) → Mg2+ (aq) + Cu(s)
ii
«+0.34V – (–2.37V) = +»2.71 «V»
iii
cell potential increases
reaction «in Q4(k)(i)» moves to the right
OR
potential of the copper half-cell increases/becomes more positive
Accept correct answers based on the Nernst equation
Examiners report
Titanium and vanadium are consecutive elements in the first transition metal series.
\({\text{TiC}}{{\text{l}}_{\text{4}}}\) reacts with water and the resulting titanium(IV) oxide can be used as a smoke screen.
Describe the bonding in metals.
Titanium exists as several isotopes. The mass spectrum of a sample of titanium gave the following data:
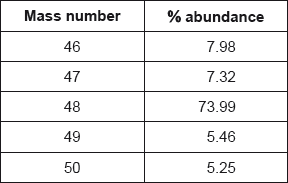
Calculate the relative atomic mass of titanium to two decimal places.
State the number of protons, neutrons and electrons in the \(_{{\text{22}}}^{{\text{48}}}{\text{Ti}}\) atom.
State the full electron configuration of the \(_{{\text{22}}}^{{\text{48}}}{\text{T}}{{\text{i}}^{2 + }}\) ion.
Suggest why the melting point of vanadium is higher than that of titanium.
Sketch a graph of the first six successive ionization energies of vanadium on the axes provided.
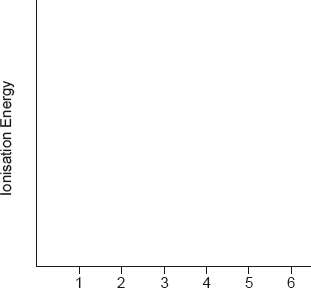
Explain why an aluminium-titanium alloy is harder than pure aluminium.
Describe, in terms of the electrons involved, how the bond between a ligand and a central metal ion is formed.
Outline why transition metals form coloured compounds.
State the type of bonding in potassium chloride which melts at 1043 K.
A chloride of titanium, \({\text{TiC}}{{\text{l}}_{\text{4}}}\), melts at 248 K. Suggest why the melting point is so much lower than that of KCl.
Formulate an equation for this reaction.
Suggest one disadvantage of using this smoke in an enclosed space.
Markscheme
electrostatic attraction
between «a lattice of» metal/positive ions/cations AND «a sea of» delocalized electrons
Accept “mobile electrons”.
Do not accept “metal atoms/nuclei”.
[2 marks]
\(\frac{{(46 \times 7.98){\text{ + }}(47 \times 7.32){\text{ + }}(48 \times 73.99){\text{ + }}(49 \times 5.46){\text{ + }}(50 \times 5.25)}}{{100}} = 47.93\)
Answer must have two decimal places with a value from 47.90 to 48.00.
Award [2] for correct final answer.
Award [0] for 47.87 (data booklet value).
[2 marks]
Protons: 22 AND Neutrons: 26 AND Electrons: 22
[1 mark]
\({\text{1}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{s}}^{\text{2}}}{\text{3}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{d}}^{\text{2}}}\)
[1 mark]
vanadium has smaller ionic radius «leading to stronger metallic bonding»
Accept vanadium has «one» more valence electron«s» «leading to stronger metallic bonding».
Accept “atomic” for “ionic”.
[1 mark]
regular increase for first five AND sharp increase to the 6th
A log graph is acceptable.
Accept log plot on given axes (without amendment of y-axis).
Award mark if gradient of 5 to 6 is greater than “best fit line” of 1 to 5.
[1 mark]
titanium atoms/ions distort the regular arrangement of atoms/ions
OR
titanium atoms/ions are a different size to aluminium «atoms/ions»
prevent layers sliding over each other
Accept diagram showing different sizes of atoms/ions.
[2 marks]
pair of electrons provided by the ligand
Do not accept “dative” or “coordinate bonding” alone.
[1 mark]
partially filled d-orbitals
«ligands cause» d-orbitals «to» split
light is absorbed as electrons transit to a higher energy level «in d–d transitions»
OR
light is absorbed as electrons are promoted
energy gap corresponds to light in the visible region of the spectrum
colour observed is the complementary colour
[4 marks]
ionic
OR
«electrostatic» attraction between oppositely charged ions
[1 mark]
«simple» molecular structure
OR
weak«er» intermolecular bonds
OR
weak«er» bonds between molecules
Accept specific examples of weak bonds such as London/dispersion and van der Waals.
Do not accept “covalent”.
[1 mark]
\({\text{TiC}}{{\text{l}}_{\text{4}}}{\text{(l)}} + {\text{2}}{{\text{H}}_{\text{2}}}{\text{O(l)}} \to {\text{Ti}}{{\text{O}}_{\text{2}}}{\text{(s)}} + {\text{4HCl(aq)}}\) correct products
correct balancing
Accept ionic equation.
Award M2 if products are HCl and a compound of Ti and O.
[2 marks]
HCl causes breathing/respiratory problems
OR
HCl is an irritant
OR
HCl is toxic
OR
HCl has acidic vapour
OR
HCl is corrosive
Accept TiO2 causes breathing
problems/is an irritant.
Accept “harmful” for both HCl and TiO2.
Accept “smoke is asphyxiant”.
[1 mark]
Examiners report
Tin(II) chloride is a white solid that is commonly used as a reducing agent.
(i) State why you would expect tin(II) chloride to have a similar lattice enthalpy to strontium chloride, using section 9 of the data booklet.
(ii) Calculate the molar enthalpy change when strontium chloride is dissolved in water, using sections 18 and 20 of the data booklet.
(iii) Tin(II) chloride reacts with water to precipitate the insoluble basic chloride, Sn(OH)Cl.
Suggest why tin(II) chloride is usually dissolved in dilute hydrochloric acid.
Tin can also exist in the +4 oxidation state.
Vanadium can be reduced from an oxidation state of +4 to +3 according to the equation:
(i) Calculate the cell potential, EΘ, and the standard free energy, ΔGΘ, change for the reaction between the VO2+ and Sn2+ ions, using sections 1 and 2 of the data booklet.
EΘ:
ΔGΘ:
(ii) Deduce, giving your reason, whether a reaction between Sn2+(aq) and VO2+(aq) would be spontaneous.
Outline, giving the full electron configuration of the vanadium atom, what is meant by the term transition metal.
In an aqueous solution of vanadium(III) chloride, the vanadium exists as [V (H2O)6]3+, [VCl (H2O)5]2+ or [VCl2(H2O)4]+ depending on the concentration of chloride ions in the solution.
(i) Describe how Cl− and H2O bond to the vanadium ion.
(ii) Outline what would happen to the wavelength at which the vanadium complex ions would absorb light as the water molecules are gradually replaced by chloride ions, using section 15 of the data booklet.
Eight successive ionisation energies of vanadium are shown in the graph below:
(i) State the sub-levels from which each of the first four electrons are lost.
First: Second: Third: Fourth:
(ii) Outline why there is an increase in ionization energy from electron 3 to electron 5.
(iii) Explain why there is a large increase in the ionization energy between electrons 5 and 6.
(iv) Vanadium is comprised almost entirely of 51V. State the number of neutrons an atom of 51V has in its nucleus.
Markscheme
(i)
same charge AND same/similar ionic radius
(ii)
enthalpy of hydration «= −1483 + 2 (−359)» = −2201 «kJmol−1»
enthalpy of solution «= 2170 − 2201» = −31 «kJmol−1»
Award [2] for correct final answer.
Award [1 max] for +31 «kJmol−1».
Award [1 max] for ±4371.
(iii)
hydrochloric acid shifts equilibrium to left
OR
hydrochloric acid prevents the basic chloride forming/precipitating
Accept “hydrochloric acid reacts with «basic» chloride” OR “hydrochloric acid suppresses salt hydrolysis”.
(i)
EΘ «= 0.34 − 0.15» = 0.19«V»
∆GOΘ«= − nFEΘ = −2 × 96500 × 0.19» = −36670 / −37000«J» / − 37«kJ»
Accept −18335 «J» or −18 «kJ» as equation not specified.
(ii)
yes AND ∆GΘ is negative
OR
yes AND EΘ for the cell is positive
OR
yes AND Sn2+ (aq) is a stronger reducing agent than V3+(aq)
OR
yes AND EΘ SN4+ (aq) is more negative that EΘ or VO2+ (aq)
OR
yes AND VO2+ (aq) is a stronger oxidizing agent than Sn4+ (aq)
OR
yes AND EΘ for VO2+ (aq) is more positive than EΘ for SN4+ (aq)
Do not accept reference to anti-clockwise rule.
1s22s22p63s23p63d34s2
OR
1s22s22p63s23p64s23d3
incomplete d «sub-» level/orbital/shell «in its compounds/ions»
(i)
give/donate a lone/non-bonding electron pair
Accept “through the formation of a dative/ coordinate bond”.
Accept “by acting as Lewis bases”.
Do not accept “act as ligands”.
(ii)
«more chlorido ligands» smaller energy gap between split d-orbitals
OR
Cl− is lower than H2O in spectrochemical series
OR
Cl− is a weaker ligand/has lower charge density
the absorption will move to longer wavelengths
OR
the absorption wavelength will increase
Do not accept answers in terms of change of frequency.
(i)
First: 4s AND Second: 4s AND Third: 3d AND Fourth: 3d
Do not apply ECF from (c).
(ii)
«in the same sub-shell and a» decrease in electron-electron repulsion
OR
«in the same sub-shell and» as more electrons removed, the pull of of the nucleus/positive ions holds the remaining electrons more tightly
Do not accept “greater nuclear charge/ effective nuclear charge”.
(iii)
electron 5 is lost from the 3d orbital
OR
electron 5 is lost from the valence shell
electron 6 is lost from a 3p orbital
OR
electron 6 is lost from a «complete» inner shell
3p orbital/complete inner shell experiences a much larger effective nuclear charge
OR
3p orbital/complete inner shell is less well shielded
OR
3p orbital/complete inner shell is nearer the nucleus
Award [1 max] (for M1/M2) (ECF) if candidate recognises electrons 5 and 6 are from different levels.
(iv)
28